
£Ø10·Ö£© Ė®ŹĒÉśĆüµÄŌ“ČŖ£¬Ņ²ŹĒ²»æÉȱɣµÄ׏Ō“”£
£Ø1£©”¢Ä³æóČŖĖ®µÄÖ÷ŅŖæóĪļÖŹ³É·Ö¼°ŗ¬ĮæČēĻĀ£ŗCa”Ŗ20 mg/L£¬K”Ŗ3 mg/L£¬F”Ŗ0.02 mg/L”£
ÕāĄļCa”¢K”¢FŹĒÖø £ØŃ”Ģī”°µ„ÖŹ”¢ŌŖĖŲ”¢·Ö×Ó”¢Ō×Ó”±£©”£
£Ø2£©”¢Ēė²ĪÓėĢÖĀŪĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
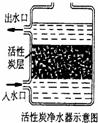
£Ø¢ń£©×ŌĄ“Ė®æɾ»»ÆĪŖŅūÓĆĖ®£¬ĘäÖŠ“¦Ąķ²½ÖčČēĻĀĶ¼ĖłŹ¾£ŗ
¢Ł¶ŌÓ¦µÄ×÷ÓĆŹĒ £ØĢī×ÖÄøŠņŗÅ£¬ĻĀĶ¬£©£¬¢Ū¶ŌÓ¦µÄ×÷ÓĆŹĒ ”£
A£®É±¾śĻū¶¾ B£®Īüø½ŌÓÖŹ C£®³Įµķ¹żĀĖ D£®ÕōĮó
£ØII£©ĻĀĮŠ×ö·Ø²»»įŌģ³ÉĖ®ĢåĪŪČ¾µÄÓŠ ”£
A£®¹¤Ņµ·ĻĖ®Ö±½ÓÅÅ·Å B£®¹¤Ņµ·ĻĘų“¦ĄķŗóÅÅ·Å
C£®½ūÖ¹Ź¹ÓĆŗ¬Į×Ļ“ŅĀ·Ū D£®“óĮæŹ¹ÓĆ»Æ·Ź”¢Å©Ņ©
£Ø¢ó£©ÄÜČ·ČĻĖ®ŹĒÓÉŃõŌŖĖŲŗĶĒāŌŖĖŲ×é³ÉµÄŹµŃéŹĒ ”£
| A£®Ė®µÄµē½ā | B£®Ė®µÄÕō·¢ | C£®ĒāĘųŌŚŃõĘųÖŠČ¼ÉÕÉś³ÉĖ® | D£®Ė®µÄ¾»»Æ |
£Ø1£©ŌŖĖŲ £Ø2£©B A BC AC £Ø3£©·ŹŌķĖ®
£Ø4£©Ė®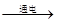 ĒāĘų+ŃõĘų 2.5ml»ņ10ml
ĒāĘų+ŃõĘų 2.5ml»ņ10ml
½āĪöŹŌĢā½āĪö£ŗ£Ø1£©Ä³æóČŖĖ®ÖŠµÄ”°Ca”¢K”¢Zn”¢F”±µČ²»ŹĒŅŌµ„ÖŹ”¢·Ö×Ó”¢Ō×ӵȊĪŹ½“ęŌŚ£¬ÕāĄļĖłÖøµÄ”°Ca”¢K”¢Zn”¢F”±ŹĒĒæµ÷“ęŌŚµÄŌŖĖŲ£¬Óė¾ßĢåŠĪĢ¬ĪŽ¹Ų£®
£Ø2£©£Ø¢ń£©»īŠŌĢæ¾ßÓŠĪüø½ŠŌ£¬ÄÜĪüø½Ė®ÖŠÉ«ĖŲ¼°ÓŠŅģĪ¶µÄŌÓÖŹ£¬Ņņ“ĖŌŚ×ŌĄ“Ė®µÄ¾»»ÆÖŠ»īŠŌĢæµÄ×÷ÓĆĪŖĪüø½×÷ÓĆ£»×ĻĶāĻßÓŠĻū¶¾É±¾śµÄ×÷ÓĆ£®¹ŹĢī£ŗB A£»
£Ø¢ņ£©A£®¹¤Ņµ·ĻĖ®Ö±½ÓÅÅ·Å£¬ÄÜŌģ³ÉĖ®µÄĪŪČ¾£» B£®¹¤Ņµ·ĻĘų“¦ĄķŗóÅÅ·Å£¬æÉÓŠŠ§µŲ½µµĶ“óĘųĪŪČ¾£»C£®½ūÖ¹Ź¹ÓĆŗ¬Į×Ļ“ŅĀ·Ū£¬ÄÜ·ĄÖ¹Ė®Ō“µÄĪŪČ¾£» D£®“óĮæŹ¹ÓĆ»Æ·Ź”¢Å©Ņ©£¬¶ŌĖ®Ō“ĪŪČ¾½ĻŃĻÖŲ£¬¹ŹĢī£ŗBC£»
£Ø¢ó£©ÄÜČ·ČĻĖ®ŹĒÓÉŃõŌŖĖŲŗĶĒāŌŖĖŲ×é³ÉµÄŹµŃéŅŖøł¾ŻÖŹĮæŹŲŗć¶ØĀÉ£¬·“Ó¦Ē°ŗóŌŖĖŲÖÖĄą²»±ä£¬ĒāĘųŌŚŃõĘųÖŠČ¼ÉÕÉś³ÉĖ®ŗĶĖ®ĶصēÉś³ÉŃõĘųŗĶĒāĘų£¬¶¼ÄÜĖµĆ÷Ė®ÖŠŗ¬ÓŠĒāŌŖĖŲŗĶŃõŌŖĖŲ£¬¹ŹĢī£ŗAC£»
£Ø3£©Éś»īÖŠ³£ÓĆ·ŹŌķĖ®Ą“Ēų·ÖÓ²Ė®ŗĶČķĖ®£¬ÅŻÄ¶ąµÄĪŖČķĖ®£¬ÅŻÄÉŁµÄĪŖÓ²Ė®£»
£Ø4£©Ė®ŌŚĶصēĢõ¼žĻĀ·Ö½āÉś³ÉĒāĘųŗĶŃõĘų£¬¹Ź·“Ó¦±ķ“ļŹ½ĪŖĖ® ĒāĘų+ŃõĘų£»Éś³ÉĒāĘųŗĶŃõĘųµÄĢå»żĪŖ2£ŗ1£¬ÓÉ“ĖĢāÄæÖ»øųĢå»żĆ»ÓŠĖµĆ÷¾ßĢåµÄĘųĢ壬¹ŹÓ¦ĢīĪŖ2.5ml»ņ10ml”£
ĒāĘų+ŃõĘų£»Éś³ÉĒāĘųŗĶŃõĘųµÄĢå»żĪŖ2£ŗ1£¬ÓÉ“ĖĢāÄæÖ»øųĢå»żĆ»ÓŠĖµĆ÷¾ßĢåµÄĘųĢ壬¹ŹÓ¦ĢīĪŖ2.5ml»ņ10ml”£
æ¼µć£ŗŌŖĖŲµÄøÅÄī£»Ė®µÄ¾»»Æ£»Ė®µÄµē½āŹµŃ飻Ė®×ŹŌ“µÄĪŪČ¾Óė·ĄÖĪ£»“æ¾»ĪļŗĶ»ģŗĻĪļµÄÅŠ±š£®
µćĘĄ£ŗ±¾ĢāÄŃ¶Č²»“󣬵«×ŪŗĻŠŌ½ĻĒ棬ÕĘĪÕŌŖĖŲÓėĪ¢¹ŪĮ£×Ó¼°ĪļÖŹµÄĒų±š”¢“æ¾»ĪļŗĶ»ģŗĻĪļµÄĒų±š”¢Ė®µÄ¾»»Æ”¢Ė®ĢåĪŪČ¾µČÖŖŹ¶ŹĒÕżČ·½ā“š±¾ĢāµÄ¹Ų¼ü£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 32”¢Ė®ŹĒÉśĆüµÄŌ“ČŖ£¬Ņ²ŹĒ²»æÉȱɣµÄ׏Ō“£®
32”¢Ė®ŹĒÉśĆüµÄŌ“ČŖ£¬Ņ²ŹĒ²»æÉȱɣµÄ׏Ō“£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ė®ŹĒÉśĆüµÄŌ“ČŖ£¬Ņ²ŹĒ²»æÉȱɣµÄ׏Ō“£®
Ė®ŹĒÉśĆüµÄŌ“ČŖ£¬Ņ²ŹĒ²»æÉȱɣµÄ׏Ō“£®| ³É·Ö | Ca | K | Zn | F |
| ŗ¬Įæ£Ømg/L£© | 20 | 3 | 0.06 | 0.02 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ³É·Ö | Ca | K | Mg | Na |
| ŗ¬Įæ£Ømg/L£© | ”Ż4 | ”Ż0.4 | ”Ż0.5 | ”Ż0.8 |

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ¹āÕÕ |
| øßĪĀ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com