
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ���������/g | 25 | 25 | 25 | 25 |
| �ձ�����ʢ����������/g | 181.5 | m | 228.6 | 253.6 |
���� ��1�����������غ㶨�ɼ������ɶ�����̼��������
��2�����ݵ�һ�����������ٵ�����Ϊ��158g+25g-181.5g=1.5g�������μ�������������Ȼ���٣����Եڶ��μ��������������Ҳ����1.5g��m=181.5g+25g-1.5g=205g���з�����
��3��������ȫ��Ӧ�����ų�������̼��������������Ʒ��̼���Ƶ���������̼������������Ʒ�����ȼ�����Ʒ��̼����������������������ע�Աȣ��ж��Ƿ�ﵽ��ע����
��� �⣺��1�����������غ㶨�ɿ�֪��ȫ��Ӧ��ų�������̼������Ϊ��158g+100g-253.6g=4.4g��
��2����һ�����������ٵ�����Ϊ��158g+25g-181.5g=1.5g�������μ�������������Ȼ���٣����Եڶ��μ��������������Ҳ����1.5g��m=181.5g+25g-1.5g=205g��
��3������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g
$\frac{106}{x}$=$\frac{44}{4.4g}$
x=10.6g
��Ʒ��̼���Ƶ���������Ϊ��$\frac{10.6g}{12g}$��100%��88.3%��96%����˲�Ʒ���ϸ�
�ʴ�Ϊ����1��4.4��
��2��205��
��3�����ϸ�
���� ���б���ļ����У�����Ҫ���ݱ������������������������ó���������Ҫ�����ʵ�������Ȼ�������û�ѧ����ʽ���м��㣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
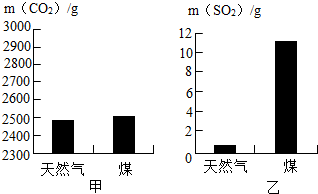
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2 | B�� | H2S | C�� | SO2 | D�� | SO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����Һ�� | B�� |  �μ�Һ�� | C�� |  �������� | D�� |  �ⶨ��ҺpH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

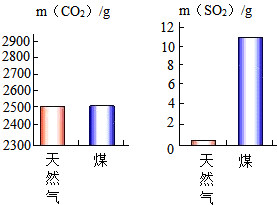
 ���͡�
���͡� ���������仯֮��Ϊ1��5��
���������仯֮��Ϊ1��5���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
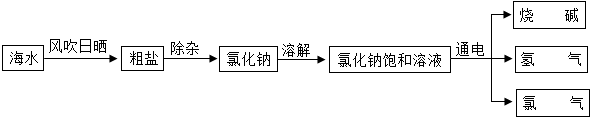
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com