
 ČēĶ¼ŹĒŅ»øö»ÆŗĻĪļµÄ×é³É·ÖĪöŹµŃ飮ŹµŃé½į¹ūČēĻĀ±ķŹ¾£ŗ²£Į§¹ÜÖŹĮæĪŖ80.2æĖ£®ŹŌøł¾ŻŹµŃ鏿¾Ż»Ų“š£ŗ
ČēĶ¼ŹĒŅ»øö»ÆŗĻĪļµÄ×é³É·ÖĪöŹµŃ飮ŹµŃé½į¹ūČēĻĀ±ķŹ¾£ŗ²£Į§¹ÜÖŹĮæĪŖ80.2æĖ£®ŹŌøł¾ŻŹµŃ鏿¾Ż»Ų“š£ŗ| ŹµŃéĒ° | ŹµŃéŗó | |
| £ØĶµÄŃõ»ÆĪļ+²£Į§¹Ü£©µÄÖŹĮæ | 137.8æĖ | 131.4æĖ |
| £ØĀČ»ÆøĘ+UŠĪ¹Ü£©µÄÖŹĮæ | 100.8æĖ | 108£®OæĖ |
| 8 |
| 64 |
| 1 |
| 16 |
| 8 |
| 64 |
| 1 |
| 16 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ŠņŗÅ | ·“Ó¦Ē°AµÄÖŹĮæ | ·“Ó¦Ē°BµÄÖŹĮæ | ·“Ó¦ŗóA3B2µÄÖŹĮæ |
| ¢Ł | 8g | 2g | 6g |
| ¢Ś | 4g | 6g | 6g |
| ¢Ū | xg | yg | 9g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ½ĖÕŹ”ÄĻ¾©ŹŠ½ÄžĒųÖŠæ¼Ņ»Ä£»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢ½¾æĢā
»Æѧ֊֏ĮæŹŲŗć¶ØĀÉŹĒŅ»øö·Ē³£ÖŲŅŖµÄ¶ØĮæ¶ØĀÉ”£
£Ø1£©ČēĻĀĶ¼×°ÖĆÖŠ£¬³ĘĮ抔ÉÕ±ÖŠĖłÓŠĪļÖŹµÄÖŹĮæm1£¬Č»ŗ󽫊”ÉÕ±ÖŠµÄĢ¼ĖįÄĘÓėŃĪĖįĶźČ«»ģŗĻ£¬·“Ó¦·¢ÉśŅ»¶ĪŹ±¼äŗó£¬ŌŁ³ĘĮ抔ÉÕ±¼°ÉÕĘæÄŚĪļÖŹµÄ×ÜÖŹĮæĪŖ m2£¬Ōņ£ØĢī”°=”± ”°£¾”±”°£¼”±£©m1 m2£¬ĘäŌŅņĪŖ ”£ 
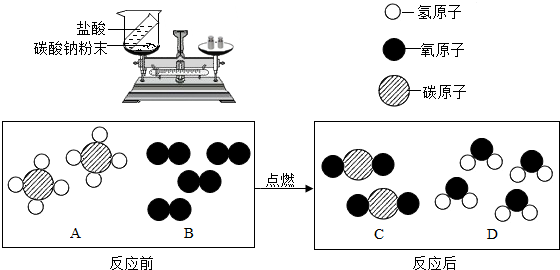
£Ø2£©ĢģČ»ĘųµÄÖ÷ŅŖ³É·ÖŹĒ¼×Ķé£ØCH4£©£¬æÉÖ±½ÓÓĆ×÷ĘųĢåČ¼ĮĻ”£Č¼ÉÕ·“Ó¦Ē°ŗó·Ö×ÓÖÖĄą±ä»ÆµÄĪ¢¹ŪŹ¾ŅāĶ¼ČēĻĀĖłŹ¾”£
¢Ł1øöB·Ö×ÓÖŠŗ¬ÓŠ øöŌ×Ó”£
¢ŚĖÄÖÖĪļÖŹÖŠŹōÓŚ»ÆŗĻĪļµÄŹĒ £ØĢīĶ¼ÖŠ×ÖÄø£©”£
£Ø3£©AŗĶBæÉ·¢ÉśČēĻĀ·“Ó¦£ŗ3A+2B=A3B2£¬Ä³Ń§Éś×öĮĖ3“ĪøĆŹµŃé£ØĆæ“Ī¾ł³ä·Ö·“Ó¦£©£¬·“Ó¦Ē°AŗĶBµÄÖŹĮæ ŗĶ¶¼ŹĒl0g”£ÓŠ¹ŲŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
ŠņŗÅ ·“Ó¦Ē°AµÄÖŹĮæ ·“Ó¦Ē°BµÄÖŹĮæ ·“Ó¦ŗóA3B2µÄÖŹĮæ
¢Ł 8g 2g 6g
¢Ś 4g 6g 6g
¢Ū xg yg 9g
X”ĆYµÄ±ČÖµæÉÄÜĪŖ »ņ ”£
£Ø4£©ĪŖĮĖ²ā¶ØÄ³Ę·ÅĘŹ³ÓĆ“æ¼īÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Ä³Š£»Æѧъ¾æŠŌѧĻ°Š”×éµÄĢ½¾æ¹ż³ĢČēĻĀ£ŗ
”¶Ģį³öĪŹĢā”·ŃłĘ·ÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹżŹĒ¶ąÉŁ?
”¶ÖŖŹ¶×¼±ø”·Ź³ÓĆ“æ¼īµÄÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįÄĘ£¬ĮķĶā»¹ŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘ£»·“Ó¦¹ż³ĢÖŠ²»æ¼ĀĒĖ®ŗĶĀČ»ÆĒāµÄ»Ó·¢”£
”¶Éč¼Ę·½°ø”·£Ø1£©·½°øŅ»£ŗĻņŅ»¶ØĮæѳʷ֊¼ÓČė¹żĮæ³ĪĒåŹÆ»ŅĖ®£¬øł¾Ż·“Ӧɜ³ÉĢ¼ĖįøʵÄÖŹĮ棬ĻČĒó³öĢ¼ĖįÄʵÄÖŹĮ棬ŌŁ¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”£
£Ø2£©·½°ø¶ž£ŗĻņŅ»¶ØĮæѳʷ֊¼ÓČė×ćĮæµÄĻ”ŃĪĖį£¬øł¾Ż·“Ӧɜ³É¶žŃõ»ÆĢ¼µÄÖŹĮ棬ĻČĒó³öĢ¼ĖįÄʵÄÖŹĮ棬ŌŁ¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”£
”¶½ųŠŠŹµŃé”·¼××éĶ¬Ń§£ŗ³ĘČ”24£®00gѳʷ£¬¼ÓĖ®Åä³ÉČÜŅŗ£¬ŌŚČÜŅŗÖŠ¼ÓČė¹żĮæµÄ³ĪĒåŹÆ»ŅĖ®”£¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬¹²µĆµ½°×É«³Įµķ20£®00g”£
ŅŅ×éĶ¬Ń§£ŗ³ĘČ”24£®00gѳʷ£¬¼ÓČė×ćĮæµÄĻ”ŃĪĖįÖ±µ½·“Ó¦Ķ£Ö¹£¬¹²ŹÕ¼Æµ½8£®80g¶žŃõ»ÆĢ¼”£
”¶½ā¾öĪŹĢā”·ĒėÄćČĪŃ”Ņ»×éĶ¬Ń§µÄŹµŃé½į¹ū£¬°ļÖśĖūĆĒ¼ĘĖć³öѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”££ØŠ“³ö¼ĘĖć¹ż³Ģ”£¼ĘĖć½į¹ū¾«Č·µ½0.1% £©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ŠņŗÅ | ·“Ó¦Ē°AµÄÖŹĮæ | ·“Ó¦Ē°BµÄÖŹĮæ | ·“Ó¦ŗóA3B2µÄÖŹĮæ |
| ¢Ł | 8g | 2g | 6g |
| ¢Ś | 4g | 6g | 6g |
| ¢Ū | xg | yg | 9g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
»Æѧ֊֏ĮæŹŲŗć¶ØĀÉŹĒŅ»øö·Ē³£ÖŲŅŖµÄ¶ØĮæ¶ØĀÉ”£
£Ø1£©ČēÓŅĶ¼×°ÖĆÖŠ£¬³ĘĮ抔ÉÕ±ÖŠĖłÓŠĪļÖŹµÄÖŹĮæm1£¬Č»ŗ󽫊”ÉÕ±ÖŠµÄĢ¼ĖįÄĘÓėŃĪĖįĶźČ«»ģŗĻ£¬·“Ó¦·¢ÉśŅ»¶ĪŹ±¼äŗó£¬ŌŁ³ĘĮ抔ÉÕ±¼°ÉÕĘæÄŚĪļÖŹµÄ×ÜÖŹĮæĪŖ m2£¬Ōņ£ØĢī”°=”± ”°£¾”±”°£¼”±£©m1 m2 £¬ĘäŌŅņĪŖ ”£

£Ø2£©ĢģČ»ĘųµÄÖ÷ŅŖ³É·ÖŹĒ¼×Ķé£ØCH4£©£¬æÉÖ±½ÓÓĆ×÷ĘųĢåČ¼
ĮĻ”£Č¼ÉÕ·“Ó¦Ē°ŗó·Ö×ÓÖÖĄą±ä»ÆµÄĪ¢¹ŪŹ¾ŅāĶ¼ČēĻĀĖłŹ¾”£

¢Ł1øöB·Ö×ÓÖŠŗ¬ÓŠ øöŌ×Ó”£
¢ŚĖÄÖÖĪļÖŹÖŠŹōÓŚ»ÆŗĻĪļµÄŹĒ £ØĢīĶ¼ÖŠ×ÖÄø£©”£
£Ø3£©AŗĶBæÉ·¢ÉśČēĻĀ·“Ó¦£ŗ3A+2B=A3B2£¬Ä³Ń§Éś×öĮĖ3“ĪøĆŹµŃé£ØĆæ“Ī¾ł³ä·Ö·“Ó¦£©£¬·“Ó¦Ē°AŗĶBµÄÖŹĮæ![]() ŗĶ¶¼ŹĒl0g”£ÓŠ¹ŲŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
ŗĶ¶¼ŹĒl0g”£ÓŠ¹ŲŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
| ŠņŗÅ | ·“Ó¦Ē°AµÄÖŹĮæ | ·“Ó¦Ē°BµÄÖŹĮæ | ·“Ó¦ŗóA3B2µÄÖŹĮæ |
| ¢Ł | 8g | 2g | 6g |
| ¢Ś | 4g | 6g | 6g |
| ¢Ū | xg | yg | 9g |
X”ĆYµÄ±ČÖµæÉÄÜĪŖ »ņ ”£
£Ø4£©ĪŖĮĖ²ā¶ØÄ³Ę·ÅĘŹ³ÓĆ“æ¼īÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Ä³Š£»Æѧъ¾æŠŌѧĻ°Š”×éµÄĢ½¾æ¹ż³ĢČē
ĻĀ£ŗ
”¾Ģį³öĪŹĢā”æѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżŹĒ¶ąÉŁ?
”¾ÖŖŹ¶×¼±ø”æŹ³ÓĆ“æ¼īµÄÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįÄĘ£¬ĮķĶā»¹ŗ¬ÓŠÉŁĮæµÄĀČ»ÆÄĘ£»·“Ó¦¹ż³ĢÖŠ²»æ¼ĀĒĖ®ŗĶĀČ»ÆĒāµÄ»Ó·¢”£
”¾Éč¼Ę·½°ø”æ£Ø1£©·½°øŅ»£ŗĻņŅ»¶ØĮæѳʷ֊¼ÓČė¹żĮæ³ĪĒåŹÆ»ŅĖ®£¬øł¾Ż·“Ӧɜ³ÉĢ¼ĖįøʵÄÖŹĮ棬ĻČĒó³öĢ¼ĖįÄʵÄÖŹĮ棬ŌŁ¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”£
£Ø2£©·½°ø¶ž£ŗĻņŅ»¶ØĮæѳʷ֊¼ÓČė×ćĮæµÄĻ”ŃĪĖį£¬øł¾Ż·“Ӧɜ³É¶žŃõ»ÆĢ¼µÄÖŹĮ棬ĻČĒó³öĢ¼ĖįÄʵÄÖŹĮ棬ŌŁ¼ĘĖćѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”£
”¾½ųŠŠŹµŃé”æ¼××éĶ¬Ń§£ŗ³ĘČ”24£®00gѳʷ£¬¼ÓĖ®Åä³ÉČÜŅŗ£¬ŌŚČÜŅŗÖŠ¼ÓČė¹żĮæµÄ³ĪĒåŹÆ»ŅĖ®”£¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬¹²µĆµ½°×É«³Įµķ20£®00g”£
ŅŅ×éĶ¬Ń§£ŗ³ĘČ”24£®00gѳʷ£¬¼ÓČė×ćĮæµÄĻ”ŃĪĖįÖ±µ½·“Ó¦Ķ£Ö¹£¬¹²ŹÕ¼Æµ½8£®80g¶žŃõ»ÆĢ¼”£
”¾½ā¾öĪŹĢā”æĒėÄćČĪŃ”Ņ»×éĶ¬Ń§µÄŹµŃé½į¹ū£¬°ļÖśĖūĆĒ¼ĘĖć³öѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹż”££ØŠ“³ö¼ĘĖć¹ż³Ģ”£¼ĘĖć½į¹ū¾«Č·µ½0.1% £©
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com