
����Ŀ��������dz��õ��մ���ϴҺ,С��ͬѧ��ijƷ�ƽ�������Ч�ɷּ��京�������о���
��1���������ϵ�֪:��������Ч�ɷ���HCl,HCl�ĺ�����ͨ����֪��������������NaHCO3��Һ���ⶨ,�����ɷ־������뷴Ӧ�������HCl��NaHCO3��Ӧ�Ļ�ѧ����ʽ:HCl+NaHCO3=NaCl+___+CO2����
��2����һ��ƿ�м���100 g��Ʒ�ƵĽ����,����μ�����ͬ����������NaHCO3��Һ,���ÿ����ƿ�з�Ӧ����Һ��������,���ݼ�¼����:
��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | |
����NaHCO3��Һ������/g | 40 | 40 | 40 | 40 | 40 |
��Ӧ����Һ��������/g | 138.9 | 177.8 | 216.7 | 255.6 | 295.6 |
�� ��________��ǡ����ȫ��Ӧ��
�� ��Ʒ�ƽ������HCl�����������Ƕ���?________
���𰸡�H2O �� 3.65%
��������
��1��HCl��NaHCO3��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��HCl+NaHCO3=NaCl+H2O+CO2����
��2���ٵ�һ�����Ĵμ���40g̼��������Һ��������1.1g�Ķ�����̼������μ���40g̼������û�в���������̼���ʵ��Ĵ�ǡ����ȫ��Ӧ��
����:��100 g��Ʒ�ƽ������HCl��������x��

x=3.65 g
�������HCl������������![]() ��
��
��:��Ʒ�ƽ������HCl������������3.65%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������֮Դ.
��1������̿�����ھ�ˮ,��������____________________ ������ˮ�е�����.
��2�����ˮʵ����ͼ��ʾ����ֱ����Դ���������IJ����������ɵ�������_____�����A�Թ����ռ���16ml�����壬��B�Թ����ռ���_____ml�����壬��Ӧ�Ļ�ѧ����ʽΪ ____________________ ��

��3������500g���ʵ���������Ϊ10����������Һ����ˮ������Ϊ ____________________ g����Ҫ�����г�������ȡ��_________________��װƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ�ס������ֹ������ʵ��ܽ�����ߣ�����˵����ȷ����

A��20��ʱ�������ʵ��ܽ�ȱ������ʵ��ܽ��С
B���������л������������ʣ����������ᾧ�ķ����ᴿ������
C��40��ʱ���ס����������ʵı�����Һ�����������������
D��60��ʱ����80g�����ʷ���100g ˮ�У�������Һ�������������ܼ�����֮���� 4:5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������װ�ã������Ҫ��ش�����:
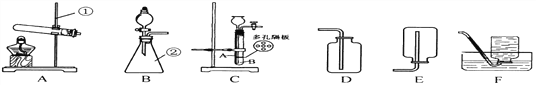
(1)д���б�������ڵ�����:______________________��ʵ������Aװ����ȡ�����Ļ�ѧ����ʽ:______________________��
(2)���Bװ�õ������Եķ�����:��ס�Ҳർ���ܣ�____________________�����Һ©���м�������ˮ�����۲쵽��Һ©��ĩ�˳����ȶ���ˮ������װ�ò�©����
(3)����װ��C��ȡ������̼���壬Ϊ�˷�ֹ������̼�ӳ���©���¶��ݳ�����ȷ�IJ�����:_________________�����п�״�������ʯӦ������__________________��(����A������B��)��һ��ʱ�����ֹͣ��Ӧ���������______________________��
(4)ʵ�����ø��������ȡ������������֤����ȼ�ղ����ʵ�飬���ѡ���ռ�װ��Ϊ________________�����)������жϸ�װ�����ռ�������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������������ڷ�Ӧǰ���������ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
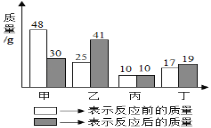
A. ��һ���ǻ�����
B. �÷�Ӧ�ǻ��Ϸ�Ӧ
C. ��һ���Ǹ÷�Ӧ�Ĵ���
D. ��Ӧǰ����ұ仯��������Ϊ9��8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ڿ�ݷ��������ǧ����,������ͼ��ʾ�Ķ�����ʾ��ͼ�ش��������.

(1)���������������������ڽ������ϵ���_____(�����).
(2)��ͭ����Դ���������ͭ��_____�ԡ�
(3) ���ƶ�������ķ�������������ʵ���е�_____������
(4)д���Ƚ�ͭ������ԵĻ�ѧ����ʽ_____
(5)�Ͼɵ�����Ҫ���ⶪ����Ӧ�������ã���������������_____.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ˣ��ҵĹ��������ˣ��ҵĹ�����! 2017�꣬����һ�ųɹ��Խӣ����ɼ������Ƶġ�ֱһ19E��ֱ�����ɳɹ������л���Ů�������帴�˵ĵ�·�ϡ�ߣ�����Ӽ���!��

��1��ֱ������ȼ���Ǻ���ú�ͣ���________���ƵIJ�Ʒ��ֱ���ɻ����չ����е�����ת��Ϊ��___________�����ܡ�____________��
��2�������մɲ��Ͽ��������մɷ�������ͨ�����õIJ����ǵ���
�裨Si3N4�����û������й�Ԫ�صĻ��ϼ�Ϊ_______��
��3�����Ͻ��̼��ά���ϲ����Ƿɻ�������ij��ò��ϣ�����Ҫ��
�������Ǿ��еĹ�ͬ������______������ĸ����
A���ܶ�С��ǿ�Ⱥ� B���ܶȴ�Ӳ�ȴ� C���۵�͡�Ӳ��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017��5��5�գ��������Ϳͻ�C919�ɳɹ�����ͼ��ʾ����ʵ���й����չ�ҵ�ش���ʷͻ�ơ��ش��������⣺

��1��ͼ�б�ʾ�IJ����У�������______________����ϳɲ��ϡ��������ϡ�����
��2���������Ϳͻ�C919���ۺϼ��ӵ���ϵͳ�ĺ��IJ��������еĵ���оƬ���Ʊ��õ���оƬ����Ҫ�����ǹ衣�赥������ʯ�ṹ���ƣ��ɹ赥�ʵ�������_________________������ţ���
��ԭ�� �ڷ��� ������
��3���ԭ�ӣ�Li���ṹʾ��ͼΪ![]() ����ʧȥ������һ�����ӣ�������ӷ�����________��
����ʧȥ������һ�����ӣ�������ӷ�����________��
��4���ڿ����У����������и��õĿ���ʴ�ԣ�ԭ����____________________���ѧ����ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2016��6��8�գ����ʴ�����Ӧ�û�ѧ���ϻ���ʽ����113�ŵ�������Ԫ�ص�Ӣ�����ƺ�Ԫ�ط��š�2017��5��9�գ��ҹ�������������Ԫ�ص��������ơ�����113��Ԫ�ص�Ԫ�ط���ΪNh����������Ϊ���b����n��������ͼ�У���Ϊ�bԭ�ӽṹʾ��ͼ����Ϊ��ԭ�ӽṹʾ��ͼ����ش���������:

��1������X=__________��
��2���ڻ�ѧ��Ӧ�У��bԭ���γ����ӵķ���Ϊ__________��
��3�������b�����Ľṹ���������b�Ľ�����Ա���ǿ����Ԥ���b��������Щ���ʷ�Ӧ����д�����ֲ�ͬ�������ʵĻ�ѧʽ���ɣ�_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com