

���� ��1����������������������һ������Һ�IJ��裬���з������
��2������ȡ�ù���ҩƷ�ķ������з������
ʹ��������ƽ����ҩƷʱӦ��ѭ���������롱������ʱ��ƽָ��ƫ��˵��ҩƷ�������������������ݴ˽��з������
������������=��Һ���������ʵ������������ɸ�����Һ�����������ʵ�������������������Һ����Ҫ�����ʵ��������ٸ����ܼ�����=��Һ����-���������������ˮ��������
����E�������ܽ�������ɲ����������ý��з������
��3����������������С�����������������ƫС���ܼ�����ƫ���Է��������������������������ԭ����з������
��� �⣺��1������50g������������Ϊ5%��ʳ����Һ�����ȼ���������Һ����ʳ�κ�ˮ���������ٳ��������ʳ�κ���ȡˮ���������ܽ⡢װƿ��ǩ��
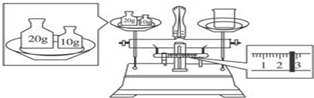
��2��A������ƿ��û�е��ţ�ijѧ����������ƽ����ʳ�Σ������ǣ�������ƽƽ�⣬�������ϼ����룬Ȼ���������������ʳ��ֱ����ƽƽ�⣻����ʱ��ƽָ��ƫ��˵��ʳ�ε�������������������Ӧ����ʳ�Σ�
��������=��Һ���������ʵ���������������50g������������Ϊ5%��ʳ����Һ�����Ȼ��Ƶ�����=50g��5%=2.5g���ܼ�����=��Һ����-����������������ˮ������=50g-2.5g=47.5g����47.5mL����
E�������ܽ�������������������ǽ��裬�ӿ�ʳ�ε��ܽ����ʣ�
��3������B�����У������ʳ�εķ��õߵ���1g���������룩�������ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС��
��C�������й����������ձ����棬�����ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС��
����ȡˮ�����ʱ�����Ӷ�����������ʵ��Һ�����С�������ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС��
��D������������ˮ�����������ʵ����ȡ��ˮ�����ƫС����ʹ������������ƫ��
����Һ���о�һ�ԣ������ƺõ���Һת�Ƶ��Լ�ƿʱ����Һ�彦�������������������䣮
��NaCl�к��п��������ʣ������ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС��
�ʴ�Ϊ����1�����㡢�������ܽ⡢װƿ��ǩ��
��2��ƿ��û�е��ţ�����ʳ�Σ�2.5g��47.5�����裬�ӿ�ʳ�ε��ܽ����ʣ�
��3���٢ڢۢޣ�
���� �����ѶȲ�����������������������һ������Һ�Ļ������衢ע��������������������йؼ��������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ֱ����̼���� | B�� | �ֱ����п�� | ||
| C�� | �ֱ�����Ȼ�����Һ | D�� | �ֱ�����̪��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧ����ʽ | ��Ӧ���� | |
| A | H2O$\frac{\underline{\;ͨ��\;}}{\;}$H2+O2�� | �ֽⷴӦ |
| B | 2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2�� | �ֽⷴӦ |
| C | Fe+2HCl�TFeCl2+H2�� | ���Ϸ�Ӧ |
| D | Fe+O2�TFe3O4 | ���Ϸ�Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú̿��ʯ��ʯ�����ڲ���������Դ | |
| B�� | Ϊ�������������ԣ���ᳫȫ����ú | |
| C�� | ú̿��ʯ��ʯ������������Ҫԭ�� | |
| D�� | Ϊ���������Դ���ᳫ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com