
��2010��������ɽ��21����ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ�������ʵ�����Ҫ�� �÷ֲ����ġ��������ʵ��װ��ͼ�ش����⣺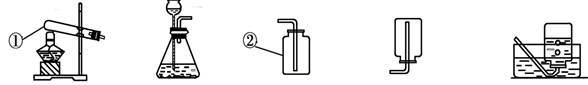
A B C D E
(1)д��ָ�����������ƣ��� ���� ��
(2)д��ʵ�������ù������⡢�����������;����ȡ�����Ļ�ѧ����ʽ
�� ��
(3)ʵ�����ø��������ȡ����ʱ����ѡ�õķ���װ���� (����ĸ����)������ˮ���ռ�������Ϻ�ֹͣ����ʱ�IJ���˳������ ��Ȼ�� ���Է�ֹˮ���������ȵ��Թ��У�����Թ����ѡ�
(4)ʵ������ȡ������̼ʱ����ѡ�õķ������ռ�װ���� (����ĸ����)����Ӧ����ʽ�� ��
����ø�װ����ȡ���壬��Ӧ���нϳ�ʱ���ȼ�ŵĻ����ڼ���ƿ�ڣ������Բ�Ϩ���ԭ������� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2010��������ɽ��21��(5��)������ͼ��ʾʵ��װ�ûش����⣺
A B C D E
(1)д��������������ƣ��� ���� ��
(2)����װ��A��ȡ��һ������Ļ�ѧ��Ӧ����ʽΪ ��������ռ�װ���� (�����)��
(3)����װ��B��ȡ��һ������Ļ�ѧ��Ӧ����ʽΪ ��������ռ�װ���� (�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2010��������ɽ��17�������ճ������г��õĹ��ߣ����е��������������⡣
(1)���������������ʹ��������?
(2)�����ĸ�������ͨ����ȡ�������ķ����ʩ?(����������ͬ��λ�IJ�ͬ��ʩ)
(3)��������������û�ѧ�Լ���ȥ����д������ķ�Ӧ����ʽ ��
(4)��о������ת�������������м� ʹ����ת����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2010��������ɽ��2��2009�������������ڸ籾�������С��й��������ڼ��ŵij�ŵ�����չʾ���й�ı��չ���ٺ����������εĴ������Ŀǰ������̼������Ϊ����Ĺ�ʶ��
(1)CO2�������࣬����ЧӦ��ǿ��
����ʮ������������CO2�����������Ҫԭ���� ��
(2)���ٴ����ж�����̼�������о���
�ٽ������еĶ�����̼���͵������������ء�������̼����е��ܽ�ȱ�ͨ���������ˮ�е��ܽ�ȴ�ԭ���� �� ���п�ѧ�ҵ��������������Ӻ�ˮ����ȣ����º�������������������̼ʹ��ˮ��� ���ӵ�ԭ���� (�û�ѧ����ʽ��ʾ)��
�ڽ�����Ķ�����̼�������ڴ����ͼ��ȵ������·�Ӧ��ת��Ϊˮ�ͼ��顣�����Ӧ�Ļ�ѧ����ʽΪ ��
(3)���ܼ��ţ���̼���
�ٽ���3��28�հ�ɽ��������������ˡ�����һСʱ���������Ϩ��һСʱ������˵������������ҪĿ�IJ�����ϵ��� ��
A����Լ��Դ �� B���������������ŷ� C�����ͳ���ҹ�� D����עȫ������
�ڵ�̼�����ϸ���������һ����̼�ٴ�������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| | ʵ��l | ʵ��2 | ʵ��3 | ʵ��4 |
| ������ĩ���� | 2��84g | 4��26g | 6��23g | 7��5lg |
| ����H2������ | 0��10g | 0��15g | 0��20g | 0��20g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ȫ���п���ѧ����������20������Һ���ܽ�� ���ͣ������
��2010��������ɽ��16���ס������ֹ������ʵ��ܽ����������ͼ��ʾ���ֽ���֧�ֱ�װ�мס��������ʱ�����Һ���Թ�(�ײ���������δ�ܽ�Ĺ���)����ʢ��ˮ���ձ��У������ձ��м���һ����Ũ���ᡣ

(1)50��ʱ�������ʵ��ܽ����������������
(2)30��ʱ���ס����������ʵ��ܽ�ȴ�С˳������������������������������
(3)�ձ��м���Ũ����ס������Թ��й������ı仯�� �����˼�Ũ�����⣬�����Լ� ���ʣ�Ҳ�ᷢ�������ı仯��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com