
���� ��Ҫ��Ϥ�������������ơ���;��ʹ�÷�����
��������ڶ������̵Ĵ������£����ȷֽ������Ȼ��غ�������
������������ˮ���ܶȱȿ�����
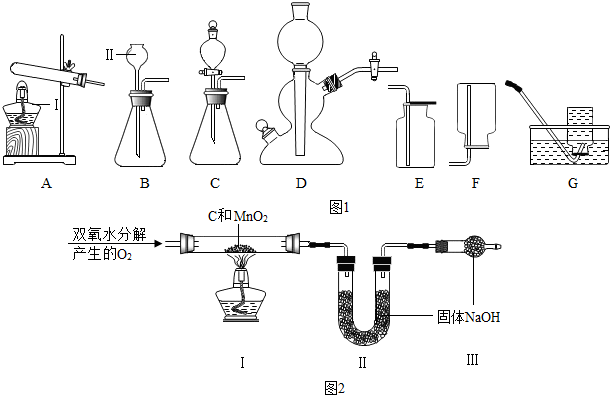
�ۿ��������շ�������Ϊ����װ�õ������ǣ������Һ�巴Ӧ������Ҫ���ȣ������ǿ���״���״�ģ�
��п��ϡ���ᷴӦ��������п�����������ݷ�Ӧ�Ļ�ѧ����ʽ���ṩ�����ݿ��Խ�����ط���ļ��㣻
��ͨ������£����������ڶ������̵Ĵ������£��ֽ�����ˮ��������
̼��������Ӧ���ɶ�����̼��
��� �⣺�٢��Ǿƾ��ƣ����dz���©����
���壺�ƾ��ƣ�����©����
��������غͶ������̵Ļ������ȡ������Ҫ���ȣ�Ӧ����Aװ����Ϊ����װ�ã�
��ȡ�ϸ����������ѡ����ռ�װ����E��
���AE��
��a��˫��ˮ��������̷�ĩ�����ȡ����ʱ�����ڶ��������Ƿ�ĩ�����������շ�������ȡ��
b��ʵ������ȡ������̼ʱ������ʯ��ʯ��ʯ�ǿ�״�ģ�ϡ������Һ�壬����Ҫ���ȣ����������շ�������ȡ��
c����п����ϡ������ȡ����ʱ��п���ǿ���״�ģ�ϡ������Һ�壬����Ҫ���ȣ����������շ�������ȡ��
���bc��
�ܽ⣺����Ҫп�������ʵ���Ϊx��
Zn+H2SO4=ZnSO4+H2����
1 1
x 0.02mol
$\frac{1}{x}$=$\frac{1}{0.02mol}$��
x=0.02mol��
п������Ϊ��0.02mol��65g/mol=1.3g��
����Ҫп��������1.3g��
����⣺����Ҫп�������ʵ���Ϊx��
Zn+H2SO4=ZnSO4+H2����
1 1
x 0.02mol
$\frac{1}{x}$=$\frac{1}{0.02mol}$��
x=0.02mol��
п������Ϊ��0.02mol��65g/mol=1.3g��
����Ҫп��������1.3g����
�ݢ�ʵ���У���˫��ˮ��������̻����ȡ�����Ļ�ѧ����ʽ�ǣ�2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
Ϊ�õ�ƽ�ȵ�������ѡ�õķ���װ����C����ΪC�еķ�Һ©���ܹ����Ʒ�Ӧ���ʣ�
���2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����C��
��a��֪����Ӧǰ����������װ�õ����������ܼ���������̵ĺ�����
b����Ӧǰ��װ�â��й���������Ϊ̼������������̼�������Լ������������������һ�����Լ��������������������
c����Ӧǰ��װ�â��й���������Ϊ��Ӧ���ɶ�����̼���������ݶ�����̼�������Լ���̼�����������������������������������
d��֪����Ӧǰ��װ�â��й�������������ܼ���������̵�����������
���b��
���� �������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������


 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| AQg | ����������� | PM2.5 | PM10 | CO | O2 | NO2 | SO2 |
| 687 | �ض���Ⱦ | 144 | 694 | 6 | 20 | 24 | 26 |
| A�� | ��ֹ���ʺ�ũҩ��ʹ�� | B�� | �о������ʯȼ�Ϻ�����Դ | ||
| C�� | ��Ч��չ�˸����ֻ | D�� | �з�������Ⱦ������¼��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȼ����ȼ�գ�˵��ˮ���п�ȼ�� | |
| B�� | ��ȼ���ķ���Ϊ�ҹ���������ʹ�ø�Ч����Դ�����˹�����ǰ�� | |
| C�� | ��ȼ��CH4•nH2O���ڱ� | |
| D�� | �������ÿ�ȼ�����ṩ��ˮ���ܱ���͵�֤�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +2 | B�� | +3 | C�� | +4 | D�� | +6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ca��H2PO4��2���� | B�� | �ɱ�������ʳƷ | ||
| C�� | ����������ȼ�� | D�� | �������ƶ���;���Դ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ�����õ������Ǵ����� | |
| B�� | ��Ӧ��������ԭ�����������ʵ���֮��Ϊ3��4 | |
| C�� | �μӷ�Ӧ��CO��O2�����ʵ���֮��Ϊ3��2 | |
| D�� | ��Ӧ���������C��Oԭ�ӵ����ʵ���֮��Ϊ3��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̬ˮ�γɳ��ٽ�ˮ�Ĺ����ǻ�ѧ�仯 | |
| B�� | ���ٽ�ˮ�ķ��Ӳ����˶� | |
| C�� | ���ó��ٽ�ˮ������������ɫ��Ⱦ�����й㷺��Ӧ��ǰ�� | |
| D�� | ���ٽ�ˮ��һ�����͵Ļ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ | B�� | â��ե��֭ | C�� | ��������ɾ� | D�� | ����֯�ɲ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com