

���� ��1������ȼ�ϵ����ʽ��н��
��2������Ӳˮ�к��н϶�����Եĸơ�þ�������Լ�����̿���������Խ��н��
��3���������ʵ������Լ����ɵ��Ȼ�����������ˮ�γ����ỹ���Ժ�������þ��Ӧ���н��
��4�����������غ㶨�ɣ�������̼���������Ƿ�Ӧ����ٵ�����������̼���Ƶ������������ɸ��ݼ��ٵĶ�����̼�����������Ʒ��̼���Ƶ�������������Ʒ��̼���Ƶ������������ɣ�
��� �⣺��1����A����Ȼ�����ڲ���������Դ����A����
B������ȼ�ϱȹ���ȼ�������ʸ��ߣ���B��ȷ��
C����Ȼ����Ϊȼ��Ҳ���ɶ�����̼�����Բ��ɱ�������ЧӦ�ķ�������C����
D��ú��������ȼ���յ��˷���Դ����������ӹ�����D��ȷ��
��2����Ӳˮ��ָ���н϶�����Եĸơ�þ�������ˮ�������跨��ȥӲˮ�еĸơ�þ���������ʹӲˮ��������ˮ��
�ڹ������������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��Ҫ���������ã�
��3����A���������̼��Ƹ����������������ƣ������ƺ�ˮ��Ӧ�����������ƣ�����ͨ��һ����Ӧ����ʵ�֣� B���������Ŀ���ǴӺ�ˮ���ᴿŨ�ȸ��ߵ��Ȼ�þ��C����������ǵ���ת��Ϊ��ѧ�ܣ� D���ڴ��������漰�Ļ�����Ӧ������3�֣�û���û���Ӧ��
�����ɵ��Ȼ�����������ˮ�γ����ỹ���Ժ�������þ��Ӧ�������ڴ������п���ѭ�����õ��������Ȼ��⣻
��4�����ٸ��������غ㶨�ɣ����ɵĶ�����̼�������������������ٵ�����������12g+72.4g-80g�T4.4g��
������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g
$\frac{106}{x}=\frac{44}{4.4g}$
x�T10.6g
������Ʒ��̼���Ƶ���������Ϊ����100%�T88.3%
�����ɶ�����̼������Ϊ4.4g��
��Ʒ��̼���Ƶ���������Ϊ88.3%
�𰸣�
��1��D��
��2���ơ�þ�����������Ե�Ca2+��Mg2+���� ������
��3����B�� ���Ȼ��⣻
��4�������ɶ�����̼������Ϊ4.4g�� ����Ʒ��̼���Ƶ����������� 88.3%��
���� ���������ϵ����ʵ�ʣ��ۺ��Խ�ǿ�������漰��ȼ�ϸ��¡���ȼ�ױ���İ�ȫ֪ʶ�Լ������غ㶨�ɵ�Ӧ�õ�֪ʶ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ױ�ѹ��--���Ӵ�С��ͬ | |
| B�� | ����Һ�������С--��������ı� | |
| C�� | ǽ�ڿ���ǽ����--���Ӳ����˶� | |
| D�� | ������̼������һ����̼��ȼ��--ԭ�����з�ʽ��ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�鲽�� | ʵ������ | ���� |
| ����ˮ���ռ���ƿ���������壬ȡ��һƿ���������壬��ƿ�еμӼ��� �����ʯ��ˮ���� | ����� | ���������ж�����̼������ |
| ʵ�鲽�� | ʵ������ | ���� |
| ��ȼ�ŵ�ľ���ֱ�����ʢ�к�������Ϳ����ļ���ƿ�� | ����ʢ��������ļ���ƿ��ȼ�ŵ�ľ����Ϩ�𣬿�����ȼ�ŵ�ľ���ճ�ȼ�� | �����������к������������� |
| ʵ�鲽�� | ʵ������ | ���� |
| �����IJ���Ƭ�Ϲ��� | ����Ƭ����һ��ˮ�� | �����������к�ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
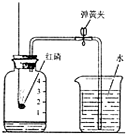 ������ˮ�����������������Ȼ��Դ��
������ˮ�����������������Ȼ��Դ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����C��D������֮��Ϊ26g | B�� | ����12gC | ||
| C�� | ��5gBʣ�� | D�� | ����10gD |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com