
һ�죬С���߽�ʵ���ң�������һ��������г���Ļ��棨����ͼ����
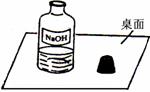 Χ�ƴ�ƿ�Լ��Ƿ���ʵ����⣬չ����̽����
Χ�ƴ�ƿ�Լ��Ƿ���ʵ����⣬չ����̽����
��1��С��������в��룺
�� ��һ������Լ���ȫ���ʣ�����Һ�������� ��
��һ������Լ���ȫ���ʣ�����Һ�������� ��
�����������Լ����ֱ��ʣ�����Һ��������NaOH��Na2CO3��
������������Լ�û�б��ʣ�����Һ��������NaOH��
��2��д��NaOH�������ʵĻ�ѧ��Ӧ����ʽ��
��
��3���������ʵ��֤ʵ���Լ��Ѿ����ֱ��ʣ����̽��������
| ʵ�鲽�� | Ԥ�������� |
|
|
��1��Na2CO3(��̼����)��1�֣���
��2��2NaOH+ CO2 == Na2CO3 + H2O��2�֣�
��3����6�֣�
| ʵ����� | ʵ�������� |
| ȡ��������Һ���Թܣ���������BaCl2��Һ��1�֣� | ������ɫ������1�֣�����˵����Һ�к���Na2CO3��1�֣� |
| ���ú�������Թ��У��μ�������̪��Һ��1�֣� | ��Һ��죨1�֣�����˵����Һ�к���NaOH��1�֣� |
����ע��������������ʵ�ʵ�鷽����ȷ����3�֡����У�û��ȡ����1�֣�BaCl2��Һû��д����������1�֣���������1�֣����۴����1�֣������ָ��֡����ʵ�鷽�������֡���
��������ѡ�Լ��������ɣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | Ԥ�������� |
ȡ��������Һ���Թܣ���������BaCl2��Һ ȡ��������Һ���Թܣ���������BaCl2��Һ |
������ɫ��������˵����Һ�к���Na2CO3 ������ɫ��������˵����Һ�к���Na2CO3 |
���ú�������Թ��У��μ�������̪��Һ ���ú�������Թ��У��μ�������̪��Һ |
��Һ��죬��˵����Һ�к���NaOH ��Һ��죬��˵����Һ�к���NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ������������п�һģ��ѧ�Ծ��������棩 ���ͣ�̽����
һ�죬С���߽�ʵ���ң�������һ��������г���Ļ��棨��ͼ����Χ�ƴ�ƿ�Լ��Ƿ���ʵ����⣬չ����̽����
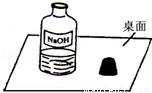
��1��С��������в��룺
����һ������Լ���ȫ���ʣ�����Һ�������� ��
�����������Լ����ֱ��ʣ�����Һ��������NaOH��Na2CO3��
������������Լ�û�б��ʣ�����Һ��������NaOH��
��2��д��NaOH�������ʵĻ�ѧ��Ӧ����ʽ��___________________________________��
��3���������ʵ��֤ʵ���Լ��Ѿ����ֱ��ʣ����̽��������
|
ʵ�鲽�� |
Ԥ�������� |
|
|
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ������������п���ѧһģ�Ծ��������棩 ���ͣ������
| ʵ�鲽�� | Ԥ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com