
 ĪŖĮĖ½ųŅ»²½ŃŠ¾æŹµŃéÖŠ³öĻÖµÄĪŹĢā£¬Č”ĮĖ13.3gĒāŃõ»ÆÄĘ¹ĢĢåѳʷ¼ÓŹŹĮæµÄĖ®Åä³ÉČÜŅŗ£¬ĻņĘäÖŠ¼ÓČė200g10%µÄĻ”ŃĪĖį£¬Ź¹Ęä³ä·Ö·“Ó¦£¬Éś³É¶žŃõ»ÆĢ¼2.2g£®Ēó£ŗ
ĪŖĮĖ½ųŅ»²½ŃŠ¾æŹµŃéÖŠ³öĻÖµÄĪŹĢā£¬Č”ĮĖ13.3gĒāŃõ»ÆÄĘ¹ĢĢåѳʷ¼ÓŹŹĮæµÄĖ®Åä³ÉČÜŅŗ£¬ĻņĘäÖŠ¼ÓČė200g10%µÄĻ”ŃĪĖį£¬Ź¹Ęä³ä·Ö·“Ó¦£¬Éś³É¶žŃõ»ÆĢ¼2.2g£®Ēó£ŗ·ÖĪö £Ø1£©ĄūÓĆ·½³ĢŹ½Na2CO3+2HClØT2NaCl+H2O+CO2”ü£¬øł¾Ż2.2æĖ¶žŃõ»ÆĢ¼µÄÖŹĮæ¼ĘĖć³öĶźČ«·“Ó¦µÄĢ¼ĖįÄʵÄÖŹĮ棻
£Ø2£©æŖŹ¼Ź±¼ÓČėµÄŃĪĖįŹĒŗĶĒāŃõ»ÆÄĘ·“Ó¦£¬²»Éś³É¶žŃõ»ÆĢ¼£»ĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦Ķź±ĻŗóŃĪĖį²ÅŹĒŗĶĢ¼ĖįÄĘ·“Ó¦£¬ÕāŹ±Éś³É¶žŃõ»ÆĢ¼½ųŠŠ½ā“š£»
£Ø3£©øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖćæÉÖŖ£¬¼ÓČėµÄŃĪĖįÖŹĮæÓėÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮæŹĒŅ»Ö±Ļß·½³Ģ£¬¾Ż“Ė»³öĒśĻߣ®
½ā“š ½ā£ŗ£Ø1£©Éčѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæĪŖx£¬Ģ¼ĖįÄĘĻūŗÄŃĪĖįµÄÖŹĮæĪŖy£®
Na2CO3+2HClØT2NaCl+H2O+CO2”ü
106 73 44
x y”Į7.3% 2.2g
$\frac{106}{x}=\frac{73}{7.3%y}=\frac{44}{2.2g}$
x=5.3g
y=50g
ѳʷ֊ĒāŃõ»ÆÄʵÄÖŹĮæ£ŗ13.3g-5.3g=8g
“š£ŗѳʷ֊ĒāŃõ»ÆÄʵÄÖŹĮæĪŖ8g£»
£Ø2£©ÉčŗĶĒāŃõ»ÆÄĘ·“Ó¦µÄĻ”ŃĪĖįµÄÖŹĮæĪŖz£®
NaOH+HClØTNaCl+H2O
40 36.5
8g z”Į7.3%
$\frac{40}{8g}=\frac{36.5}{7.3%z}$
z=100g
“š£ŗŗĶĒāŃõ»ÆÄĘ·“Ó¦µÄĻ”ŃĪĖįµÄÖŹĮæĪŖ100g£®
£Ø3£©æŖŹ¼ĒāŃõ»ÆÄĘŗĶŃĪĖį·“Ó¦²»·Å³ö¶žŃõ»ÆĢ¼£¬ĘäÖŠĒāŃõ»ÆÄĘĻūŗÄŃĪĖįµÄÖŹĮæĪŖ100g£¬Č»ŗóĢ¼ĖįÄĘŗĶŃĪĖį·“Ó¦·Å³ö¶žŃõ»ÆĢ¼£¬ĘäÖŠĢ¼ĖįÄĘĻūŗÄŃĪĖįµÄÖŹĮæĪŖ50g£¬Éś³É¶žŃõ»ÆĢ¼µÄÖŹĮæĪŖ2.2g£¬ĖłŅŌ¹ŲĻµĶ¼ĪŖ£ŗ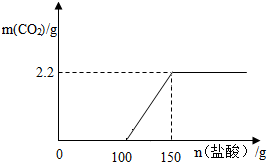 £®
£®
µćĘĄ øĆĢāÄæ¼ÆŹµŃéŗĶ¼ĘĖćÓŚŅ»Ģ壬ŹĒæ¼²éµÄÖŲµćĢāŠĶ£¬ŅŖĒóѧɜ½āĢā¹ż³ĢÖŠŅŖ×¢ŅāÖŖŹ¶¼äµÄĮŖĻµ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ£Ė®”¢¾»»ÆŗóµÄæÕĘų | |
| B£® | ŅŗŃõ”¢¹żŃõ»ÆĒāĶźČ«·Ö½āŗóŹ£ÓąŅŗĢå | |
| C£® | ÉśŠāµÄĢś¶¤”¢Ę”¾Ę | |
| D£® | ±łĖ®»ģŗĻĪļ”¢øßĆĢĖį¼Ų¼ÓČČŗóµÄ¹ĢĢåŹ£ÓąĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹÆÄ«µ¼µē | B£® | »īŠŌĢæ¾»»ÆĖ® | ||
| C£® | ÓƵ¶Ļ÷Ćę | D£® | ½«ŹÆÄ«×Ŗ»Æ³É½šøÕŹÆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com