

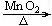 2KCl+3O2�� a
2KCl+3O2�� a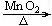 2KCl+3O2��������װ��H�ռ�����������ˮ������������Ӧ��a�ˣ����̹ܣ�����
2KCl+3O2��������װ��H�ռ�����������ˮ������������Ӧ��a�ˣ����̹ܣ�����

 �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������©�������ƿ�����ܡ��ƾ��� |
| B������©�������ƿ�����ܡ�����ƿ |
| C������©�������ƿ�����ܡ�ˮ�� |
| D�����Թܡ����ܡ���Ͳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��CuO��CO Cu��CO2 Cu��CO2 | B��NaOH��HCl NaCl��H2O NaCl��H2O |
C��3Fe��2O2 Fe3O4 Fe3O4 | D��CaCO3 CaO��CO 2�� CaO��CO 2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ���ܶȱȿ���С |
| B��������ˮ���ܶȱȿ����� |
| C��������ˮ���ܶȱȿ���С |
| D��������ˮ���ܶȱȿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ũ����ƿ�����ɿ����������� |
| B�����ڿ�����ȼ�գ���������������ɫ���� |
| C����ˮ�е�����ɫʯ����Һ����Һ���ɫ |
| D��������ʱ�䱩¶�ڳ�ʪ�Ŀ����У�������������� |
�鿴�𰸺ͽ���>>
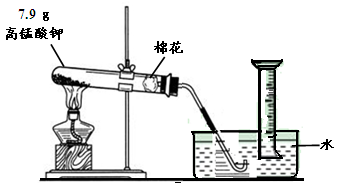
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A������װ����ͬ | B��������MnO2������ |
| C����Ӧ�Ļ���������ͬ | D����ȫ��Ӧ��ʣ�����ɷ���ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com