
���Ʋ�ϡ��һ������������Na2SO4��Һ��
��1������50g��������Ϊ6%��Na2SO4��Һ��
�ټ��㣺��ҪNa2SO4 3.0g��ˮ 47.0g
�ڳ�������������ƽ����3.0g��Na2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ����������Ȼ����������������ƽǡ��ƽ�⣮
����ȡ������Ͳ��ȡ47.0mLˮ��������ͼ�л���47.0mLˮ��Һ��λ�ã�
���ܽ⣮
��2��ϡ����Һ���������������ƹ�������Һ��ϡ�����ܶȿɽ��ƿ���1g/mL��
��ȡ1mL 6%��Na2SO4��Һ��ˮϡ����100mL���õ���Һa��
������3.0gNa2SO4��������ҺaŨ����ͬ����Һ�����������mL��
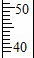
���㣺 һ������������������Һ�����ƣ�������-������ƽ��
ר�⣺ ��Һ����Һ���ܽ�ȣ�
������ ��1���ڸ��ݳ���3gҩƷ�IJ��������ش�
�۸�������Ͳ��ȡ47.0mL�IJ�������ͼʾ��
��2�����������������估��Һ���ܶȡ��������֮��Ĺ�ϵ�������㣮
��� �⣺��1������������ƽ����3.0g��N a2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ��������������3g���룬Ȼ��������������ҩƷ����������ƽǡ��ƽ�⣮
a2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ��������������3g���룬Ȼ��������������ҩƷ����������ƽǡ��ƽ�⣮
����ȡ������Ͳ��ȡ47. 0mLˮ��47.0mLˮ��Һ��λ����ͼ��
0mLˮ��47.0mLˮ��Һ��λ����ͼ��
��2������Һa�����������ǣ�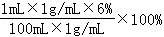 =0.06%
=0.06%
��������Һ������Ϊ�� =5000g������ǣ�
=5000g������ǣ� =5000mL
=5000mL
�ʴ�Ϊ����1��������������3g���룬������������ҩƷ���ۼ���ͼ����2��5000��

������ ������Ҫ����������һ������������Һ�Ļ����������ѶȲ����ڲ�����һ��Ҫץס������Ҫ�㣬ȷ������
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����ͨ�������仯����������ǣ�������
| �� | A�� | ˮ������ | B�� | úȼ�շ��� |
| �� | C�� | ըҩ��ը��ɽ��· | D�� | ����ȼ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��Ƽҵ������������°�̽�������ˡ������Ƽ���������������漰����Ҫ��ѧ��Ӧ���£�
��NH2��CO2��X��NH4HCO3[
��NH4HCO3��NaCl��NH4Cl��NaHCO3��
��2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ش��������⣺
��1����Ӧ����X�Ļ�ѧʽΪ��
��2����ȥ����Na2CO3��ĩ��������NaHCO3�ķ����� ��
��3����ҵ�����к����Ȼ��ƣ�ȡ55g��ҵ��������м���269.5gϡ���ᣬǡ����ȫ��Ӧ������22g������̼����
�ٹ�ҵ������̼���Ƶ�������������������������0.1%��
�ڷ�Ӧ����Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�һ���������������ƹ�������ˮ�Ƴ���Һ�������йص�������ˮ�������ı���ı���ǣ�������
�� A.��Һ�������� B.��Һ��pH
�� C.�������Ƶ��ܽ�� D.��������ն�����̼������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
3%��������Һ��������ϴƤ����С������ˣ�������300g��������Ϊ 3%��������Һ��ʵ��������£�
3%��������Һ��ʵ��������£�
��1����������������������ͼ1�зֱ�����ѡ����������������ʾ����

��2����ȡˮ�����������Ͳȡ����mLˮ����ˮ��1g/cm3����
��3���ܽ⣺�õ��IJ�������������
��4��װƿ������ǩ����ͼ2�ı�ǩ��������Ӧ�����ݣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ԭ���ǻ�ѧ�仯�е���С���ӡ��ĸ�����(����)
A��ԭ�ӵ�������С B��ԭ���ǹ������ʵ���С����
C��ԭ�ӵ������С D��ԭ���ڻ�ѧ��Ӧǰ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ����Ϣ�жϣ�����˵���������(����)
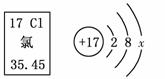
A����ԭ�ӵ���������17
B .��ԭ�Ӻ�����3�����Ӳ�
C����x��8ʱ��������������
D���ڻ�ѧ�仯�У���ԭ���õ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б仯���ǻ�ѧ�仯����(����)
A���������в������� B��ú��ʯ�͵��γ�
C��ʯ�ͷ���õ����� D��ú����õ���̿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У����ڼ���ʳ����ǣ�������
| ѡ�� | A | B | C | D |
| ʳ�� | ƻ��֭ | ����֭ | ţ�� | ������ |
| pH | 2.9��3.3 | 3.5��4.5 | 6.3��6.6 | 7.6��8.0 |
| �� | A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com