


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
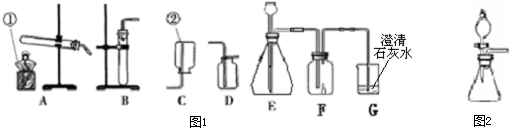
 ��2010?�Ϻ���ģ�⣩2010���Ϻ��������й���-������֮�ڡ�����ǿ�ҵ��Ӿ��������������ṹΪ�ĸ����ֽ�������Ƴɵĺ���Ͳ�����иֽ���������ڣ�������
��2010?�Ϻ���ģ�⣩2010���Ϻ��������й���-������֮�ڡ�����ǿ�ҵ��Ӿ��������������ṹΪ�ĸ����ֽ�������Ƴɵĺ���Ͳ�����иֽ���������ڣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?�Ϻ���ģ�⣩��Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա�����������һ���ж������ʣ����Ⱦ���ߵ���������˺�������ӽṹ��ͼ��ʾ�������йضԱ�������˵����ȷ���ǣ�������
��2010?�Ϻ���ģ�⣩��Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա�����������һ���ж������ʣ����Ⱦ���ߵ���������˺�������ӽṹ��ͼ��ʾ�������йضԱ�������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com