
 ���ɱ�ʾ������O2���ѧ���ţ���
���ɱ�ʾ������O2���ѧ���ţ���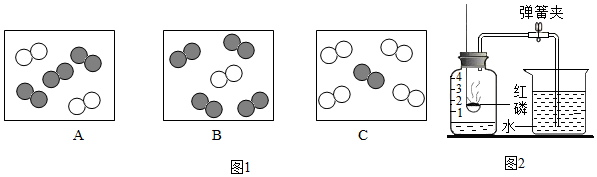
���� ��1�����ݿ�������ɷ������
��2�����ݷ�������ԭ�ӹ��ɣ������е����������ijɷֺ������
��3��A�����ݻ�ʯȼ�ϵ�ȼ�ջ����������Ⱦ�������
B�������ᳫ���С������г��ȡ���̼�����з�ʽ�����Լ��ٶ�����̼���ŷŽ��
C�������ᳫʹ��̫���ܵ������Դ�ܼ�����Ⱦ����ŷŷ�����
D�����ݻ�������ͨ���Ӹ��̴��ŷŷ��������ܷ�ֹ�γ�������
��4�����ݿ�����������������������ش�
��� �⣺��1�������к���������������������̼�����ʣ����ڻ���
��2���ٷ�������ԭ�ӹ��ɵģ��á� ���ɱ�ʾ�����������ӣ���ѧʽ�ǣ�O2��
���ɱ�ʾ�����������ӣ���ѧʽ�ǣ�O2��
������Լռ���������$\frac{1}{5}$������Լռ���������$\frac{4}{5}$�������뵪�����������1��4������ͬ��ͬѹ�£����������ȵ��ڷ�����Ŀ�ȣ���ͼ�пɱ�ʾ������ģ�͵���C��
��3��A�����ٻ�ʯȼ�ϵ�ȼ�գ��ܼ��ٿ�����Ⱦ����ŷŵȣ�����ȷ��
B���ᳫ�����ʻ˽�ҳ����У�������Ⱦ����ŷţ������ڼ��ٿ�����Ⱦ���ʴ���
C���ᳫʹ��̫���ܵ������Դ���ܼ�����Ⱦ����ŷţ�����ȷ��
D����������ͨ���Ӹ��̴��ŷŷ��������ܷ�ֹ�γ����꣬�ʴ���
��4����ʵ��̽���ĽǶȣ�����ͼ2��ʾ��ʵ����п����ɷֵIJⶨ�����ڿ������������������Լռ���������$\frac{1}{5}$��������Ϩ����ȴ���ɼУ��۲쵽���뼯��ƿ�ڵ�ˮԼռ����ƿ�ݻ���$\frac{1}{5}$��
�ʴ�Ϊ����1��������2����O2�� ��C����3��BD�� ��4��$\frac{1}{5}$��
���� ������Ҫ�����˿����ijɷ֡�������Ⱦ������ͼ����ʶ��֪ʶ���Ƚ��ۺϣ�Ҫϸ�ķ�����


 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ף��ң�п | B�� | �ң�п���� | C�� | �ң�п���� | D�� | п���ף��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1��ë���þ��˻��Ӳ��ƣ�ԭ��֮һ�ǣ���ë��ϴ��ʱ������ˮ�еĸơ�þ������������ò��������������������ᣩ������ë���ϣ�����Ҫ��ش����⣮
��1��ë���þ��˻��Ӳ��ƣ�ԭ��֮һ�ǣ���ë��ϴ��ʱ������ˮ�еĸơ�þ������������ò��������������������ᣩ������ë���ϣ�����Ҫ��ش����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
 ��ľ������ũ�ҷ��ϣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȣ�Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ100g�ò�ľ�����ձ��У����ϵ���ϡ������Һ��������30gϡ����ʱ�����������ݲ�������ʱ�ձ��еIJ�����������Ϊ127.8g��̼��������ᷴӦ�Ļ�ѧ����ʽΪK2CO3+H2SO4=K2CO4+CO2��+H2O
��ľ������ũ�ҷ��ϣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȣ�Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ100g�ò�ľ�����ձ��У����ϵ���ϡ������Һ��������30gϡ����ʱ�����������ݲ�������ʱ�ձ��еIJ�����������Ϊ127.8g��̼��������ᷴӦ�Ļ�ѧ����ʽΪK2CO3+H2SO4=K2CO4+CO2��+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����CaCl2��Na2CO3���ȼ�������ˮ��Ȼ����ˣ����� | |
| B�� | ����NaOH��Na2CO3���ְ�ɫ���壺ȡ������������ˮ���۲� | |
| C�� | ��ȥBaSO4�е�BaCO3��ȡ������������ϡ���ᣬ���ˣ�ϴ�ӣ����� | |
| D�� | �����������к���CO��CO2���Ƚ��������ͨ�����ȵ�����ͭ����ͨ��ʯ��ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com