��2009?���ţ�������������������Դ��������ˮ��Դ����ʳ�ȷ����Σ����
��1��Ŀǰ����ͨ����ѧ��Ӧ��õ��������������ú��ʯ�͡�
��Ȼ��
��Ȼ��
�Ȼ�ʯȼ�ϣ���Ϊ��ʯȼ����Դ���ޣ������������úͿ�����ϫ�ܡ������ܡ�
̫����
̫����
������Դ��
��2����ʯȼ�ϵ�ʹ�ã������ǵ���������ܶ�㣬ͬʱҲ�Ի�������˲���Ӱ�죬�磺úȼ�ջ��ŷų���������SO
2��������������NO
2������Ⱦ�SO
2��NO
2����
������
������
��ѡ����ʡ����������������
��3����������Ϊ��������ȼ�ϡ������û�ѧ����ʽ���͡�������������ԭ��
��
��4���������������Ȼˮϵ��Ⱦ��ʹ����ˮ��Դ���٣�ijЩ������ʳ�����ܵ�����Ӱ�죮��ʳ�е���Ҫ�ɷ�Ϊ���ۣ���������
����
����
��ѡ������ʡ���ά���ء�����֬�������ࡰ������������������Ҫ��Դ��




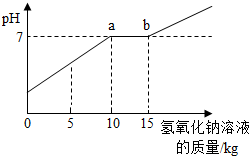 ��2009?���ţ�ͨ����ʵ�������ꡰ��������ȡ��ʵ���Ҫ����Һ�����ҺͰ�У������Ϳ��ռ�һЩ���������п�Ļ����Һ���������������ʣ���Ϊ������Ⱦ������ijУ��ѧ��ȤС���ͬѧ��������ʵ�飺
��2009?���ţ�ͨ����ʵ�������ꡰ��������ȡ��ʵ���Ҫ����Һ�����ҺͰ�У������Ϳ��ռ�һЩ���������п�Ļ����Һ���������������ʣ���Ϊ������Ⱦ������ijУ��ѧ��ȤС���ͬѧ��������ʵ�飺