2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�
��1������ˮ��Դ�������������������е���
��
A��ϴ����ˢ��ʱ���ֹر�ˮ��ͷ B����ϴ�ˡ�ϴ�µ�ˮ��������ϵ�
C����ϴ�»�ϴһ�������� D��ʹ�ý�ˮ����Ͱ
��2������ˮ��������������
�ķ�����ȥˮ�в��������ʣ��ٽ���������X��һ�ֳ���������ˮ����������ҵ��ȡX�Ļ�ѧ����ʽΪ��Cl
2+2NaClO
2=2NaCl+2X����X�Ļ�ѧʽ��
��
��3��ˮ��Ӳ�ȹ����Ӱ�����������������ˮ��Ӳˮ���õ�������
��
��4��ˮ��������ܼ�������������������ˮ��Ϻ����γ���Һ����
��
A��ֲ���� B���Ȼ��� C������ D���������
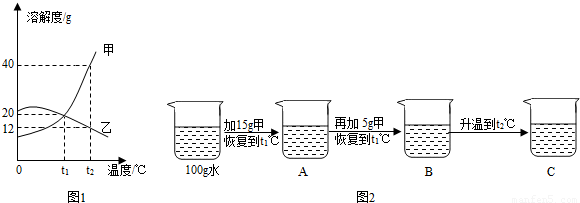
��5��ͼ1Ϊ�ס��ң��������ᾧˮ�����ֹ���������ˮ�е��ܽ�����ߣ�
���ܽ�����¶����߶������������
����ס����ҡ�����t
1��ʱ�����ܽ��
�ҵ��ܽ�ȣ�����ڡ���С�ڡ����ڡ�����
��t
2��ʱ��100gˮ���ܽ�
g������ǡ�ôﵽ���ͣ�
��ijͬѧ��t
1��ʱ��ʼ����ʵ�飬�õ�ͼ2����Ӧ����ҺA��B��C��
����ҺA��B��C�У����ڱ�����Һ����
������ҺC������ȵ���
��
����ҺC���ټ���25g��ֽ��裬�ָ���t
2�棬������Һ������Ϊ
��






 2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�
2012��3��22���ǵڶ�ʮ�조����ˮ�ա���ˮ����������������������أ�