���ࣨ��C6H126�ƣ��ڷ���ʱ��Ӧ�����Ҵ��Ͷ�����̼���仯ѧ��Ӧ����ʽΪ��C6H12O6=2C2H5OH+2CO2����������l°�ľ���ָ��1����Ҵ���99���ˮ�Ļ����Һ���ʣ�
��1����������������������Һ�����ܵ�1°�ľƣ���ˮ���Ҵ����ܶȷֱ�Ϊ1.0g/cm3��0.8g/cm3�����������һλС����
��2����Ƴ�������ʱ��������������һ��ϡ����Һ��������ȣ�������ɺ���������ɵõ��ƣ�������ѧ��ѧ֪ʶ������ΪӦ�������ϡ����Һ��������ȣ�Ϊʲô��
���𰸡�
����������ȷ����⣬����ݻ�ѧ����ʽ�ó�������֮��������ȣ��г�����ʽ��ͨ������ó����ʵ�������Ȼ�������������������ʽ���㼴�ɣ�
����⣺��1��1�Ⱦ���1����Ҵ�������Ϊ��1mL×0.8 g/cm
3=0.80g��99���ˮ������Ϊ��99mL×1 g/cm
3=99g
���ǵ�����Ϊx��
C
6H
12O
6=2C
2H
5OH+2CO
2��
180 92
x 0.8g
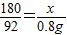
��֮�ã�x��1.6g��
������Һ������������

×100%��1.6%��
��2�����Ϸ�����ɺ��������Ĺ����ǣ��Է��ͺ�����Һ�����������Ȼ�����������������������ȴ���£�ʹ֮���Һ�壬����һ�����У�������������ϡ������Һ��������ȣ���ΪH
2SO
4�����лӷ��ԣ������ϡ���ᣬ��������лӷ��ԣ�����ʱ�����е�HCl���������ƾ��У��õ��ľƾ����������ʣ�
�ʴ�Ϊ����1��������������Ϊ1.6%��������Һ���͵õ�l°�ľƣ�
��2��Ӧ����ϡH
2SO
4��������ȣ���ΪH
2SO
4�����лӷ��ԣ�����H
2SO
4�ķе�ߣ�
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������

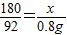
 ×100%��1.6%��
×100%��1.6%��

