
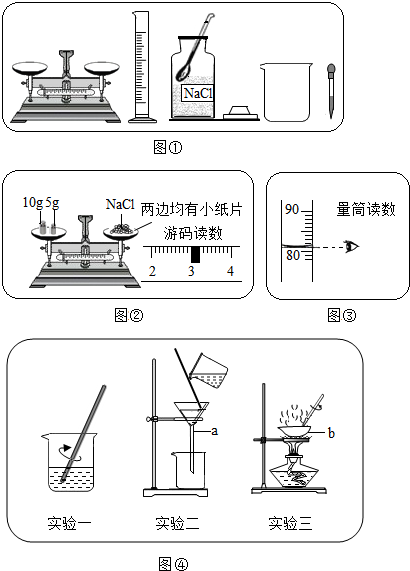
 ij»ÆѧŠĖȤŠ”×é½ųŠŠČÜŅŗÅäÖĘŗĶ“ÖŃĪ³õ²½Ģį“æŹµŃ飮
ij»ÆѧŠĖȤŠ”×é½ųŠŠČÜŅŗÅäÖĘŗĶ“ÖŃĪ³õ²½Ģį“æŹµŃ飮| ČÜÖŹÖŹĮæ |
| ČÜŅŗÖŹĮæ |
| 18g |
| 18g+82g |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢³£ÓĆÓŚŹµŃéŹŅÖĘČ”ŃõĘųµÄ·“Ó¦¶¼ŹĒ·Ö½ā·“Ó¦ |
| B”¢ÓĆÅÅĖ®·ØŹÕ¼ÆŃõĘųŹ±£¬µ¼¹ÜæŚøÕÓŠĘųÅŻĆ°³ö¾ĶŹÕ¼ÆĘųĢå |
| C”¢Įņ·ŪŌŚŃõĘųÖŠČ¼ÉÕŹ±£¬¼ÆĘųĘæÖŠ·ÅÉŁĮæĖ®æÉŅŌĪüŹÕ¶žŃõ»ÆĮņ |
| D”¢ŹµŃéŹŅÓĆ¹żŃõ»ÆĒāÖĘŃõĘųµÄ·“Ó¦ÖŠ£¬¶žŃõ»ÆĆĢĘš“ß»Æ×÷ÓĆ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢ »ģŗĻ |
B”¢ ³ÉŠĶ |
C”¢ øÉŌļ |
D”¢ ÉÕ½į |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢“ņæŖŹ¢ÓŠÅØŃĪĖįŗĶÅØĮņĖįµÄŹŌ¼ĮĘæĘæČū£¬ŌŚĘææŚ¶¼ÓŠ°×Īķ |
| B”¢½«Ź³“×µĪŌŚŹŖµÄpHŹŌÖ½ÉĻ£¬²ā¶ØĘäpH |
| C”¢“߻ƼĮŌŚ»Æѧ·“Ó¦ÖŠŅ»¶ØÄܼÓæģ»Æѧ·“Ó¦ĖŁĀŹ |
| D”¢ÓĆ·ŹŌķĖ®æÉĒų·ÖÓ²Ė®ŗĶČķĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
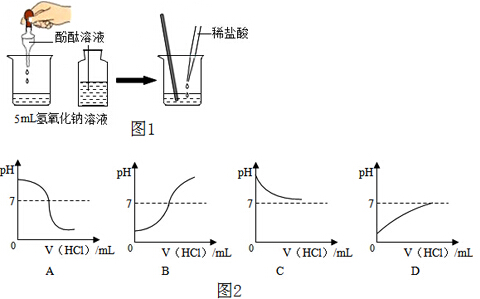
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| AӢCO2 |
| BӢCaO |
| CӢMgCl2 |
| DӢH2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢½«»ģŗĻĘųĢåµćČ¼ |
| B”¢½«»ģŗĻĘųĢåĶعż³ĪĒåŹÆ»ŅĖ® |
| C”¢¼ÓŃ¹»ņ½µĪĀŹ¹¶žŃõ»ÆĢ¼±äĪŖøɱł |
| D”¢½«»ģŗĻĘųĢåÓÉŅ»øöČŻĘ÷Ēćµ¹µ½ĮķŅ»øöČŻĘ÷Ąļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com