
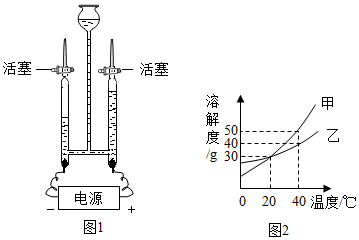
·ÖĪö £Ø1£©»īŠŌĢæ¾ßÓŠĪüø½ŠŌ£¬Äܹ»Īüø½É«ĖŲ”¢ŅģĪ¶µČ£»
£Ø2£©Éś»īÖŠ³£ÓĆ·ŹŌķĖ®Ą“Ēų·ÖÓ²Ė®ŗĶČķĖ®£»
£Ø3£©µē½āĖ®Ź±£¬Õż¼«²śÉśµÄŹĒŃõĘų£¬øŗ¼«²śÉśµÄŹĒĒāĘų£¬ŃõĘųŗĶĒāĘųµÄĢå»ż±ČŌ¼ĪŖ1£ŗ2£¬ŃõĘųÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼£»
£Ø4£©ČÜÖŹÖŹĮæ=ČÜŅŗÖŹĮæ”ĮČÜÖŹÖŹĮæ·ÖŹż£»
£Ø5£©øł¾ŻĪļÖŹµÄČܽā¶ČĒśĻßæÉŅŌ½ųŠŠĻą¹Ų·½ĆęµÄ¼ĘĖćŗĶÅŠ¶Ļ£®
½ā“š ½ā£ŗ£Ø1£©»īŠŌĢæ³£ÓĆÓŚ¾»Ė®£¬Ęä×÷ÓĆŹĒĪüø½É«ĖŲ”¢ŅģĪ¶µČ”¢¹żĀĖĖ®ÖŠµÄŌÓÖŹ£®
¹ŹĢī£ŗĪüø½É«ĖŲ”¢ŅģĪ¶µČ£®
£Ø2£©ĻņĖ®ÖŠ¼ÓČė·ŹŌķĖ®Ź±£¬Čē¹ū²śÉśµÄÅŻÄ½Ļ¶ą£¬ŹĒČķĖ®£¬Čē¹ū²śÉś“óĮæø”Ōü£¬ŹĒÓ²Ė®£¬Ņņ“ĖÉś»īÖŠ³£ÓĆ·ŹŌķĖ®Ą“Ēų·ÖÓ²Ė®ŗĶČķĖ®£®
¹ŹĢī£ŗ·ŹŌķĖ®£®
£Ø3£©µē½āĖ®µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£¬ÓėÖ±Į÷µēŌ“Õż¼«ĻąĮ¬µÄ²£Į§¹ÜÖŠµÄĘųĢåŹĒŃõĘų£¬æÉÓĆ“ų»šŠĒµÄľĢõ¼ģŃ飮
¹ŹĢī£ŗ2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü£»ÓĆ“ų»šŠĒµÄľĢõ£®
£Ø4£©ÅäÖĘ500gČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ10%µÄĘĻĢŃĢĒČÜŅŗ£¬ĘäÖŠĘĻĢŃĢĒµÄÖŹĮæĪŖ£ŗ500g”Į10%=50g£»
ŌŁ½ųŠŠ³ĘĮ攢ĮæČ””¢Čܽā£¬×īŗóĶź³É×°ĘæŗĶĢł±źĒ©£®
¹ŹĢī£ŗ50£»Čܽā£®
£Ø5£©¢Ł30”ꏱ£¬¼×µÄČܽā¶Č“óÓŚŅŅµÄČܽā¶Č£®
¹ŹĢī£ŗ“óÓŚ£®
¢Ś40”ꏱ£¬¼×µÄČܽā¶ČŹĒ50g£¬ŅŅµÄČܽā¶ČŹĒ40g£¬Į½·Ż100gĖ®ÖŠø÷¼ÓČė40g¼×”¢ŅŅ£¬ŌņŅŅµÄČÜŅŗĪŖ±„ŗĶČÜŅŗ£®
¹ŹĢī£ŗŅŅ£®
µćĘĄ ŗĻĄķÉč¼ĘŹµŃ飬æĘѧµŲ½ųŠŠŹµŃ锢·ÖĪöŹµŃ飬ŹĒµĆ³öÕżČ·ŹµŃé½įĀŪµÄĒ°Ģį£¬Ņņ“ĖŅŖѧ»įÉč¼ĘŹµŃ锢½ųŠŠŹµŃ锢·ÖĪöŹµŃ飬ĪŖѧŗĆ»ÆѧÖŖŹ¶µģ¶Ø»ł“”£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
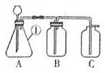 ČēĶ¼ŹĒŹµŃéŹŅÖĘČ”ĘųĢåµÄ×°ÖĆ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ŹĒŹµŃéŹŅÖĘČ”ĘųĢåµÄ×°ÖĆ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
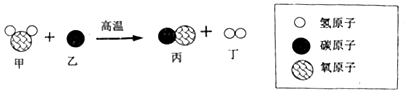
| A£® | ¼×ŗĶ±ūĮ½ÖÖĪļÖŹ¶¼ŹōÓŚŃõ»ÆĪļ | |
| B£® | øĆ·“Ó¦»ł±¾·“Ó¦ĄąŠĶĪŖÖĆ»»·“Ó¦ | |
| C£® | øĆ·“Ó¦ÖŠµÄĖÄÖÖĪļÖŹ¶¼ÓÉ·Ö×Ó¹¹³É | |
| D£® | ·“Ó¦ŗóÉś³ÉµÄĮ½ÖÖĪļÖŹµÄ·Ö×ÓøöŹż±ČĪŖ1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹµŃéÖŠŠčŅŖÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠĢģĘ½”¢ÉÕ±”¢ĮæĶ²”¢²£Į§°ōŗĶ½ŗĶ·µĪ¹ÜµČ | |
| B£® | ÅäÖĘøĆĻ”ĮņĖįŠčŅŖ¼ÓĖ®800mL | |
| C£® | ÅäÖĘĻ”ĮņĖįŹ±ÓƵ½²£Į§°ō£¬Ęä×÷ÓĆŹĒŅżĮ÷ | |
| D£® | øĆĻ”ĮņĖįÖŠ£¬ČÜŅŗÖŹĮæ£ŗČܼĮÖŹĮæ=5£ŗ4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
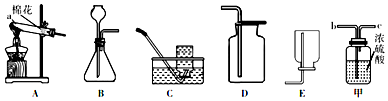
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŹĮæµÄÕōĮóĖ® | B£® | ŹŹĮæµÄĻ”ĮņĖį | C£® | ŹŹĮæµÄŹÆ»ŅĖ® | D£® | ŹŹĮæµÄĻ”ŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com