
| ||
| ||


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�걱���г��������꼶��ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ������
������ˮ�Ͷ�����̼������������Ҫ�����ʡ�
��1��A��B��C�����о�������ɺ����ʵ�ʵ�顣
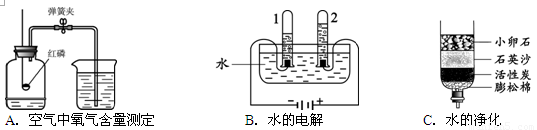
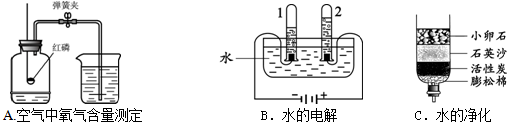
�ٹ���Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩡�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դx
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ˮ�Ͷ�����̼������������Ҫ�����ʡ�
����1��A��B��C�����о�������ɺ����ʵ�ʵ�顣
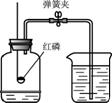
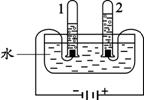

A.���������������ⶨ B��ˮ�ĵ�� C��ˮ�ľ���
�� ����Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
�� Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩡�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com