
��6�֣�Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⡣
��1����ȥ�����л��е�����������̼���壺
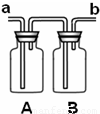
�ٳ���ʱ�������������������� ���a����b�����˽���ϴ��ƿ��
��A��B�ж�ʢ�������ij���ʯ��ˮ��B�г���ʯ��ˮ�������� ��������
����ʱ��˵��CO2�ѳ�����
��2���ø�װ����֤������̼�Ƿ���ˮ��Ӧ���ṩ��ҩƷ��
����ɫʯ����Һ ����ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ�� ����ҩƷ��ţ��������֤��������̼��ˮ�����˻�ѧ��Ӧ���÷�Ӧ��ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⣮
Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ��ɽ���������п����в��Ի�ѧ�Ծ��������棩 ���ͣ������
��6�֣�Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⡣

��1����ȥ�����л��е�����������̼���壺
�ٳ���ʱ�������������������� ���a����b�����˽���ϴ��ƿ��
��A��B�ж�ʢ�������ij���ʯ��ˮ��B�г���ʯ��ˮ�������� ��������
����ʱ��˵��CO2�ѳ�����
��2���ø�װ����֤������̼�Ƿ���ˮ��Ӧ���ṩ��ҩƷ��
����ɫʯ����Һ ����ɫʯ����ҺȾ�ɵĸ���ֽ����
A��B��Ӧ����ʢ�� ����ҩƷ��ţ��������֤��������̼��ˮ�����˻�ѧ��Ӧ���÷�Ӧ��ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⣮
Ϊ���о�������Ӽ�����֮��ķ�Ӧ���������ͼװ�÷ֱ�ʵ�飬������й����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ��ɽ���������п���ѧ�����Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com