
·ÖĪö £Ø1£©¢Łøł¾ŻŹ³Ę·ÖŠø»ŗ¬µÄÓŖŃųĖŲ½ųŠŠ½ā“š£»
¢Śøł¾ŻŹ³Ę·ÖŠø»ŗ¬µÄÓŖŃųĖŲŅŌ¼°ČĖĢå±ŲŠčµÄĮł“óÓŖŃųĖŲĄ“·ÖĪö£»
¢Ūøł¾ŻŠ”Āóø»ŗ¬µķ·Ū£¬ĢĒĄąŌŚČĖĢåÄŚ¾¹żŅ»ĻµĮŠµÄ±ä»Æ×īÖÕ×Ŗ»ÆĪŖĖ®ŗĶ¶žŃõ»ÆĢ¼½ųŠŠ·ÖĪö£»
¢Üøł¾ŻŹ³Ę·ÖŠø»ŗ¬µÄÓŖŃųĖŲŅŌ¼°ČĖĢå±ŲŠčµÄĮł“óÓŖŃųĖŲĄ“·ÖĪö£»
£Ø2£©¢Łøł¾Ż²ÄĮĻµÄ·ÖĄą½ųŠŠ·ÖĪö£»
¢Śøł¾ŻÓŠ»ś»ÆŗĻĪļµÄøÅÄī½ųŠŠ·ÖĪö£»
¢Ūøł¾ŻČ¼ÉÕŠčŅŖĶ¬Ź±Āś×ćČżøöĢõ¼ž£ŗ¢ŁæÉČ¼Īļ”¢¢ŚŃõĘų»ņæÕĘų”¢¢ŪĪĀ¶ČŅŖ“ļµ½×Å»šµć£¬ŗ¬Ģ¼ŌŖĖŲµÄČ¼ĮĻČ¼ÉÕ²»³ä·Ö»įÉś³ÉŅ»Ńõ»ÆĢ¼£¬½ųŠŠ·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¢ŁŌŚ±żøɵÄÅäĮĻÖŠ£¬ø»ŗ¬ÓĶÖ¬µÄŹĒ¾«Į¶Ö²ĪļÓĶ£»
¹ŹĢī£ŗ¾«Į¶Ö²ĪļÓĶ£»
¢ŚøĆÅäĮĻÖŠø»ŗ¬µ°°×ÖŹµÄĪļÖŹŹĒĻŹ¼¦µ°£»
¹ŹĢī£ŗĻŹ¼¦µ°£»
¢ŪŠ”Āó·ŪÖŠŗ¬ÓŠµÄĢĒĄąĪļÖŹÖ÷ŅŖŹĒµķ·Ū£¬ĢĒĄąŌŚČĖĢåÄŚ¾¹żŅ»ĻµĮŠµÄ±ä»Æ×īÖÕ×Ŗ»ÆĪŖĖ®ŗĶ¶žŃõ»ÆĢ¼£»
¹ŹĢī£ŗCO2£»
¢ÜČĖĢåŠčŅŖµÄĮł“óÓŖŃųĪļÖŹ£ŗµ°°×ÖŹ”¢ĢĒĄą”¢ÓĶÖ¬”¢Ī¬ÉśĖŲ”¢ĪŽ»śŃĪŗĶĖ®£¬“ÓÓŖŃų¾łŗāµÄ½Ē¶Čæ“£¬øĆŹ³Ę·ÖŠ³żĖ®Ķā£¬»¹Č±ÉŁµÄÓŖŃųĖŲŹĒĪ¬ÉśĖŲ£»
¹ŹĢī£ŗĪ¬ÉśĖŲ£»
£Ø2£©¢ŁĖÜĮĻŹōÓŚŗĻ³É²ÄĮĻ£»
¢Ś¶”Ķéŗ¬ÓŠĢ¼ŌŖĖŲ£¬ŹōÓŚÓŠ»ś»ÆŗĻĪļ£»
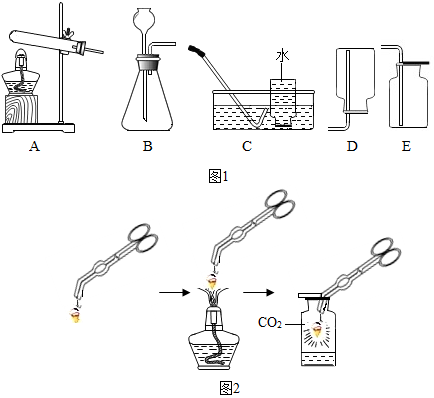
¢Ū“ņ»š»śµ²·ēÕÖĶØ·ēæ×µÄ×÷ÓĆŹĒŹ¹æÕĘųĮ÷ĶØ£¬ĖµĆ÷Č¼ĮĻČ¼ÉÕŠčŅŖÓėŃõĘų½Ó“„£»ŗ¬Ģ¼ŌŖĖŲµÄČ¼ĮĻČ¼ÉÕ²»³ä·Ö»įÉś³ÉŅ»Ńõ»ÆĢ¼£¬ČōĶØ·ēæ×ĶØĘų²»Į¼£¬æÉÄÜŌģ³É“ņ»š»śĪŽ·ØĘš»š»ņČ¼ĮĻ²»ĶźČ«Č¼ÉÕ¶ųÉś³ÉÓŠ¶¾µÄŅ»Ńõ»ÆĢ¼£¬¼ČĄĖ·ŃĮĖČ¼ĮĻÓÖĪŪČ¾ĮĖ»·¾³£»
¹ŹĢī£ŗ¢Łc£»¢ŚÓŠ»ś£»¢ŪŃõĘų£»CO£®
µćĘĄ ±¾Ģāæ¼²éµÄŹĒ»ÆѧÓėÉś»īµÄÖŖŹ¶£¬Ķź³É“ĖĢā£¬æÉŅŌŅĄ¾ŻŅŃÓŠµÄÖŖŹ¶½ųŠŠ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ¼× | ŅŅ | ±ū | ¶” | |
| ·“Ó¦Ē°µÄÖŹĮæ/g | 4 | 10 | 1 | 25 |
| ·“Ó¦ÖĮtŹ±æĢµÄÖŹĮæ/g | 2 | a | b | 17 |
| ·“Ó¦ŗóµÄÖŹĮæ/g | 0 | 22 | 9 | d |
| A£® | dµÄŹżÖµĪŖ9 | B£® | bµÄŹżÖµĪŖ4 | ||
| C£® | ¼×ŗĶ¶”ĪŖ·“Ó¦Īļ | D£® | ¶”µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ¼×µÄ2±¶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| Č”°×É«¹ĢĢåÓŚŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæĻ”ŃĪĖį | °×É«¹ĢĢåÖš½„ĻūŹ§£¬Ć»ÓŠĘųÅŻĆ°³ö | °×É«¹ĢĢå²»ŹĒMgCO3£¬¶ųŹĒMgO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Ė®ŹĒÖŲŅŖ׏Ō“£®
Ė®ŹĒÖŲŅŖ׏Ō“£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ķ | B£® | ¶žŃõ»ÆĢ¼ | C£® | ½šøÕŹÆ | D£® | ĒāŃõ»ÆÄĘ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
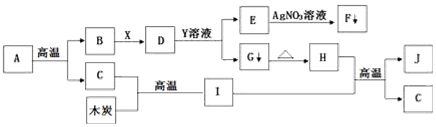
| Īļ””””ÖŹ | Ėłŗ¬ŌÓÖŹ | ³żČ„ŌÓÖŹµÄ·½·Ø | |
| A | CO2ĘųĢå | COĘųĢå | ĶØČėŃõĘų£¬µćČ¼ |
| B | CuSO4ČÜŅŗ | H2SO4ČÜŅŗ | ¼ÓČė×ćĮæCu·Ū£¬³ä·Ö·“Ó¦ŗó¹żĀĖ |
| C | C·Ū | Fe·Ū | ¼ÓČė×ćĮæŃĪĖį£¬³ä·Ö·“Ó¦ŗó¹żĀĖ |
| D | KMnO4 | MnO2 | ¼ÓČČ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com