
��ʡij���п���ѧʵ������������ĸ����⣺������һ������������������Һ���ڼ�Ļ�ѧ���ʣ���CO2����ȡ���ռ�����������O2����ȡ���ռ������������Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
��ش�
��1��ָ��ͼ1�б�����������ƣ�a b ��
��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽���ǵ� �����⣻
��3��ͼ2��С����ɸ�ʵ����Ҫ�������̵�ʾ��ͼ�������ֱ���ÿ�������ȷ��1�֣��ܹ�5�֣�ʵ����Ϻ�С�����3�֣����ҳ�С��ʧ�ֵIJ�����˵��ԭ�� �� ��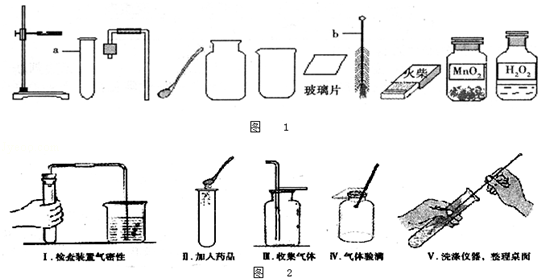
��4����������������ҩƷ��ѡ����Ҳ�������һ�ֳ��������ʵ����ȡ����ѧ����ʽΪ �������� ����һ�ֲ����������ƣ�������װ���������ȡ�����ķ���װ�ã�
��1��a���Թ�b���Թ�ˢ��2���ܣ�3������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ�����4��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
���������������1���Թ��dz��õķ�Ӧ�������Թ�ˢ��ˢ�Թܵ��������ʴ�Ϊ��a���Թ�b���Թ�ˢ��2����ʵ��̨���ṩ��������ҩƷ������ΪС��鵽����O2����ȡ���ռ����������ʴ�Ϊ���ܣ�3��С����ɸ�ʵ����Ҫ���������д�����У�����ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ������ʴ�Ϊ������ֱ���Թܿڼ���ҩƷ��ҩƷ��մ�ڹܿڻ�ܱڣ��ô�����ľ������ƿ��������������֤�Ƿ��ռ�����4��̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ������Ӿƾ��ƣ�������װ���������ȡ�����ķ���װ�ã��ʴ�Ϊ��CaCO3+2HCl=CaCl2+CO2��+H2O���ƾ���
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ������ʵ������ȡ�����ķ�Ӧԭ���������ļ������������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʵ���ҳ��õ�װ�ã������ͼ�ش��������⣺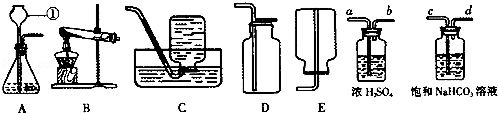
��1��д�����б�����������ƣ����� ��
��2��ʵ�����ø��������ȡ�����Ļ�ѧ����ʽ���� ������ѡ�õķ���װ������ ��������ţ���ʵ����Ϻ�Ҫ�Ƚ����ܴ�ˮ����ȡ����Ϩ��ƾ��ƣ���ԭ������
��3���ռ�ij����ֻ�ܲ���Eװ�ã��ɴ��Ʋ��������е��������� ��
��4��ʵ�����Ƶõ�CO2�����к����Ȼ����ˮ������Ϊ�˵õ������������CO2���壬����װ�õĵ���������������˳������ ������ѡ�����ţ�
| A��a��b��c��d�� | B��b��a��c��d�� | C��c��d��a��b�� | D��d��c��b��a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о���ѧϰС����������װ�ý����������ȡʵ�飬�������������
��1��ѡ��Aװ����ȡ�����Ļ�ѧ����ʽΪ ��
��2��A��E���ӣ���ȡ������������ԭ���� ��
��3�����ù���������Һ��ȡһƿ�������������Ҫ�õ�װ��F��װ��F��Ӧ����������Ǹ����Ũ���ᣬ������Ӧ�� ͨ�루��a��b��
��4��ʵ������ȡ������̼ѡ��ķ���װ�ú��ռ�װ���� ����ѡ�����ĸ����ѡ��÷���װ�õ������� �����������̼�Ƿ��ռ����ķ����� ��
��5����Ȳ��C2H2���׳Ƶ�ʯ������һ����ɫ��ζ�����壬�ܶȱȿ�����С��������ˮ��ʵ���ҳ��ÿ�״̼���ƣ�CaC2����ˮ��Ӧ��ȡ��Ȳ��ͬʱ�������������ɡ�����ͼ��ѡ��ʵ������ȡ��Ȳ��ѡ�õķ������ռ�װ���� �����ڸ÷�Ӧ�dz����ң����װ������ע�������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ���ҳ��ü�������غͶ������̻����ķ�����ȡ��������ش��������⣺
A B C D E
�ŷ�Ӧ�Ļ�ѧ����ʽΪ ��
�����������������a________________ b_______________��
��������������װ��������Ӧ��������װ�ã���ѡ��ķ���װ��Ϊ________��Bװ���Թܿ�Ҫ��
������б��ԭ����_______________________________________________���ռ�װ��Ϊ__________��
����װ��ѡ��������� ������ĸ����
| A�����ڹ̹��ͼ��ȵķ�Ӧ | B�����ڹ�Һ�Ͳ����ȵķ�Ӧ |
| C����ȡ�������ܶȱȿ����� | D����ȡ������������ˮ |

|
| ʵ����� | ��������������ص������� | ��ʱ���룩 |
| 1 | 6��6 | 42 |
| 2 | 2.5:5 | 25 |
| 3 | 2:5 | 34 |
| 4 | 3:10 | 65 |
| 5 | 2:10 | 41 |
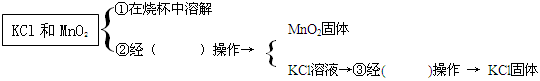
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾΪʵ�����г����������Ʊ����ռ�װ�á�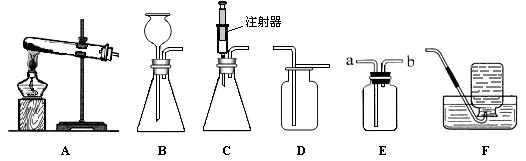
��ش��������⣺
��1��ʵ�����ø��������ȡ������Ӧѡ�÷���װ�� (����ĸ���)����Ӧ�Ļ�ѧ����ʽΪ ������װ��D�ռ������������ķ����� ��
��2��ʵ�����ù���������Һ�Ͷ���������ȡ��������ѡ��C������װ�ã�����Ϊѡ��C���ŵ��� ��
��3����ʹ��װ��E���ſ������ռ�������������Ӧ�� ���a����b������ͨ�룻��ʹ��װ��E����ˮ���ռ��������Ƚ�ƿ��װ��ˮ���ٽ������ ���a����b������ͨ�롣
��4����֪һ����������������ˮ���ڿ���������������������Ӧ�����ռ�һ����������ʱӦѡ��ͼ��װ�� (����ĸ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش����⣺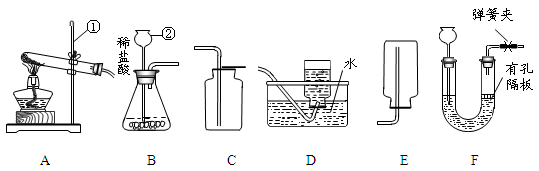
��1����Ţ٢ڵ��������ƣ�
�� ���� ��
��2��ʵ����ѡ��ADװ����ȡO2 ��д���÷�Ӧ�Ļ�ѧʽ����ʽ �������ռ����ʱ��Ӧ�� �����ˮ��ȡ�������ܡ���Ϩ��ƾ��ơ�����
��3��ʵ���ҿ���(NH4)2SO4�������ʯ�һ�ϼ�����ȡNH3��Ӧѡ����װ��Ϊ ��ѡ����ţ���
��4��ѡ����װ��B���ռ�װ�� ��ѡ����ţ���������ʵ������ȡCO2����������װ����B��ΪF�����ŵ��� ��
��5����У����ʦ����ѧ����������ʵ��ʱ�����֡�һͬѧ��������װ�������ļ���ƿ��Ѹ�ٷ����ƿ��ʣ�����������ʹ�����ǵ�ľ����ȼ�����Դˣ�����ʦ�ø�ͬѧ������ƿװ���ƿˮ��������ˮ���ռ�1/2����ƿ������������ƿ�е�����Ҳ��ʹ������ľ����ȼ��
�������з����ռ���1/2����ƿ�����������������������________������ĸ����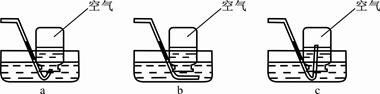
������ˮ���ռ�����ʱ������жϼ���ƿ���������ռ�����
��__________________________________________________ _________��
�ۿ������������������ԼΪ21%����ʵ���У�����ƿ�ڵ�����Լռ�������__ ____%�������á�ʹ������ľ����ȼ�������鼯��ƿ�г��������ķ������ɿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Һ�ڶ������̵���������Ѹ�ٷֽ��������ˮ����Һ©������ͨ�����ڻ�������Һ��μ��ٶȡ��ش��������⣺
��1����Һ©����Ӧ����������� ����ƿ��Ӧ����������� ��
��2��Ҫ�ռ�һƿ����������Ӧѡ����ͼ�е�װ�� ������ĸ����
��3��ijͬѧ�ڹ۲쵽��ƿ���д�������ʱ����ʼ��Cװ���ռ������� ��һ��ʱ����ô����ǵ�ľ������ƿ�ڡ�ƿ�С�ƿ�ף���δ��ľ����ȼ�������ԭ���� ��
��4��Ϊ����װ��B�������Ƿ��ռ��������ô����ǵ�ľ�����ڼ���ƿ��_______����������������_______________�����ʡ�
��5����ʵ��ʱ�ô˷����������ؼ������������ŵ��� ������ţ�
A����������ֻ������ B��������� C�������
��6��װ��A�з�Ӧ���ң��ݴ����ʵ�鰲ȫע�������� ��
A������Һ��ĵμ��ٶ� B�������С����ƿ C�����ȷ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʦΪͬѧ���ṩ������ʵ��װ�ã�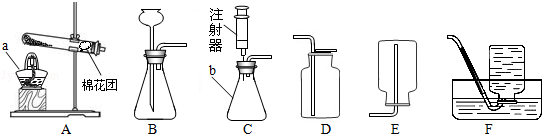
��1��д���������ƣ�a��________ ��b�� ________��
��2����װ����ʱ���������ܲ��뽺Ƥ�ܻ��������ǰ��Ҫ�ȰѲ����ܿ� ________��Ȼ������������ת��������롣
��3��ʵ����Ҫ�ø��������ȡһƿ�ϴ���������ѡ�õķ������ռ�װ����________������ĸ��ţ�����Ӧ����ʽΪ ________ ��
��4��ʵ����Ҳ����ѡ�ù���������Һ�Ͷ���������װ��B����ȡ��������Ӧ����ʽΪ ________����ѡ��C������װ�ã�����Ϊѡ��װ��C���ŵ���________��
��5��ʵ��ǰҲ������װ��B����ע�벿��ˮʹ����©���γ�Һ����ڵ��ܿ�����һ��ע���������� ________��
��6����֪һ����������������ˮ���ڿ���������������������Ӧ�����ռ�һ����������ʱӦѡ��ͼ��װ�� ________(����ĸ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͼ��ʵ���ҳ��õ���ȡ�����װ�ã��ش��й����⣺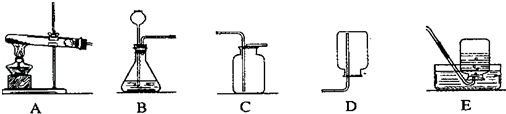
��1��Aװ�����Թܿڱ���������б��ԭ���� ��
��2����E�ռ����������߱��������� ��
��3���ø��������ȡ����Ӧѡ��ķ���װ���ǣ���װ����ţ� ��
��4��д��ʵ���ҿ���B��Cװ���������ȡ��һ�������ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com