
С���С˼ͬѧ�Ի�ѧ��������ĵá����������ǽ��һ���������ʵ¼��
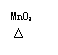 ����һ������о������������⡣����������ȷ��0.01��
����һ������о������������⡣����������ȷ��0.01��
[��Ŀ] ��֪: 2KClO3 === 2KCl + 3O2��,��10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g������������ص�������
��1��С��ܿ�õ���10g + 2g 7.2g���� ���ѧʽ�����������������KCl �������� g.
�����д��С�����KCl �������Ĺ��̡�
(2)С˼������������Ľ��������Ŀ���������⡣����ͨ���ļ��㣬��֤���ķ��֡�
��3����β��ܸ��������أ�С���С˼��Ϊ������������罫��Ŀ�С�10g����ء���Ϊ��ag����ء����������ʵ��������䣬��a��ȡֵ��Χ�� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ�������а�����ѧ���꼶���ϣ����л�ѧ�Ծ��������棩 ���ͣ������
 2KCl+3Q2������10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g���������Ȼ��ص�������
2KCl+3Q2������10g����غ�2g�������̻�Ϻ�����Թ��м��ȣ��ռ���������������ֹͣ�������Թ���ȴ���Ƶ��Թ���ʣ����������Ϊ7.2g���������Ȼ��ص������� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com