
(2011���㽭���ˣ�1��)����֪��Ԫ�����ڱ��У�ÿһ���У��壩��Ԫ�صĻ�ѧ���ʶ������ơ�
|
��A�� |
Ԫ����ɵ����� |
||
|
���� |
������ |
||
|
F |
F2 |
HF |
|
|
Cl |
Cl2 |
HCl |
HClO HClO2 HClO3 HClO4 |
|
Br |
Br2 |
HBr |
HBrO HBrO2 HBrO4 |
|
I |
I2 |
HI |
���� |
(1)���ݱ��й��ɣ��ڱ��к�����д�����ʵĻ�ѧʽ��
(2)�����к���Ԫ�ص����ʣ�����Ԫ�صĻ��ϼ۴Ӹߵ��͵�˳������
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
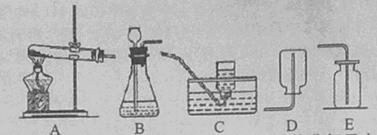
(2011���㽭���ˣ�24��)��ͼ�dz��õ����巢��װ�ú��ռ�װ�á�
��1��װ��A�����ڼ��ȷֽ���������ȡ��������ȡʱ��Ҫ���Թܿڷ�һ����������������
��2��������һ������л��ʵ���ҳ���AC��ADװ�ã����ȷֽ���ˮ�����ƺͼ�ʯ�ҵĻ��������ȡ�������塣�ɴ˿��Ƶü���������� ���������ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������4�������������Ʊ������������� ���ͣ������
(2011���㽭���ˣ�24��)��ͼ�dz��õ����巢��װ�ú��ռ�װ�á�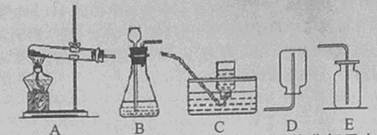
��1��װ��A�����ڼ��ȷֽ���������ȡ��������ȡʱ��Ҫ���Թܿڷ�һ����������������
��2��������һ������л��ʵ���ҳ���AC��ADװ�ã����ȷֽ���ˮ�����ƺͼ�ʯ�ҵĻ��������ȡ�������塣�ɴ˿��Ƶü���������� ���������ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п�����ר����ר������ѧ����ʽ���㣨���� ���ͣ�������
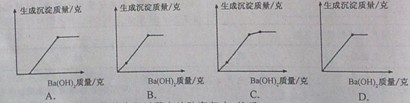
(2011���㽭���ˣ�35��)����������Һ����NaOH ��Һ�� ��Ba(OH)2��Һ����Na2SO4��Һ����ϡ���ᡢ�ݻ������������MgSO4��Һ
��1��ָ���������ڼ���Һ�� ��ѡ����ţ���
��2������Ba(OH)2��Һ��Na2SO4��Һ��ϣ���������������116.5�ˣ���Ҫ20������������Һ���ٿ�?
(3)����Ba(0H)2��Һ��μ��˵��������������MgSO4��Һ��,���ó���������������
Ba(OH)2�������仯��������ͼ����� (ѡ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п�����ר����ר������ѧ����ʽ���㣨һ�� ���ͣ������
(2011���㽭���ˣ�37��)�����ѳ�Ϊ�����˳��е���Ҫ��ͨ����,�г���ȫ��Խ��Խ�ܵ����ǵ����ӡ�������װ��ȫ���ҿ�����Ч�����˳���Ա���ڳ���������ײ��˲�䣬��ȫװ��ͨ����ʹ���еķ�ĩѸ�ٷֽ��ͷų������ĵ������γ����ң��÷�ĩ��Fe2O3����ͻ��������ɣ�����13.0�˻������,��ȫ�ֽ�����8.4�˵����͵�����.�����ҿ��ڸ��¸�����������������Fe2O3�����û���Ӧ����Na2O���ɡ�
��1��������Ļ�ѧʽ�� ��
��2��Na2O��������е�����������ͨ�����Ϸ�Ӧת��Ϊ̼�����ƣ�NaHCO3������Ӧ�Ļ�ѧ����ʽΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com