
| “Ī””Źż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ¼ÓČėĻ”ŃĪĖįÖŹĮæ/g | 15 | 15 | 15 | 15 |
| ÉÕ±ÄŚŹ£ÓąĪļÖŹĮæ/g | 19.34 | 33.68 | 48.24 | 63.24 |
| 100 |
| 44 |
| x |
| 1.76g |
| 4g |
| 5g |
| 100 |
| 44 |
| Y |
| 2.64g |
| 111 |
| 44 |
| Z |
| 2.64g |
| 6.66g |
| 63.36g |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| “Ī””Źż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | µŚĖÄ“Ī |
| ¼ÓČėĻ”ŃĪĖįÖŹĮæ/g | 15 | 15 | 15 | 15 |
| ÉÕ±ÄŚŹ£ÓąĪļÖŹĮæ/g | 19.34 | 33.68 | 48.24 | 63.24 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
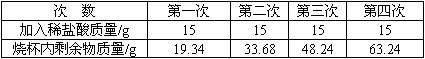
ĪŖĮĖ²ā¶ØijÖÖŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹż£¬Č”5 gŹÆ»ŅŹÆѳʷ·ÅČėÉÕ±ÖŠ£¬½«60 gĻ”ŃĪĖį·Ö³ÉĖÄ“Ī¼ÓČėÉÕ±ÖŠ£¬³ä·Ö·“Ó¦ŗ󣬲āµĆŹµŃ鏿¾ŻČēĻĀ±ķ(ѳʷ֊µÄŌÓÖŹ²»ÓėŃĪĖį·“Ó¦Ņ²²»ČÜÓŚĖ®)
ĒėÄć·ÖĪö²¢½ųŠŠÓŠ¹Ų¼ĘĖć£ŗ
(1)Äļø“Ī·“Ó¦ŗó£¬ŃĪĖįÓŠŹ£Óą___________________________________________”£
(2)ŹÆ»ŅŹÆѳʷ֊Ģ¼ĖįøʵÄÖŹĮæ·ÖŹż”£
(3)ÉĻŹöŹµŃé½įŹųŗó£¬ĻņÉÕ±ÄŚµÄĪļÖŹÖŠ¼ÓČėŹÆ»ŅŹÆÖĮ²»ŌŁ²śÉśĘųÅŻ£¬¹żĀĖ”£ĒóĀĖŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż”£(½į¹ū¾«Č·µ½0.1%)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011Äź³õÖŠ±ĻŅµÉżŃ§æ¼ŹŌ£ØÄŚĆɹŰüĶ·¾ķ£©»Æѧ ĢāŠĶ£ŗ¼ĘĖćĢā
ĪŖĮĖ²ā¶ØijÖÖŹÆ»ŅŹÆÖŠĢ¼ĖįøʵÄÖŹĮæ·ÖŹż£¬Č”5 gŹÆ»ŅŹÆѳʷ·ÅČėÉÕ±ÖŠ£¬½«60 gĻ”ŃĪĖį·Ö³ÉĖÄ“Ī¼ÓČėÉÕ±ÖŠ£¬³ä·Ö·“Ó¦ŗ󣬲āµĆŹµŃ鏿¾ŻČēĻĀ±ķ(ѳʷ֊µÄŌÓÖŹ²»ÓėŃĪĖį·“Ó¦Ņ²²»ČÜÓŚĖ®)
ĒėÄć·ÖĪö²¢½ųŠŠÓŠ¹Ų¼ĘĖć£ŗ
(1)Äļø“Ī·“Ó¦ŗó£¬ŃĪĖįÓŠŹ£Óą___________________________________________”£
(2)ŹÆ»ŅŹÆѳʷ֊Ģ¼ĖįøʵÄÖŹĮæ·ÖŹż”£
(3)ÉĻŹöŹµŃé½įŹųŗó£¬ĻņÉÕ±ÄŚµÄĪļÖŹÖŠ¼ÓČėŹÆ»ŅŹÆÖĮ²»ŌŁ²śÉśĘųÅŻ£¬¹żĀĖ”£ĒóĀĖŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹż”£(½į¹ū¾«Č·µ½0.1%)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗÄŚĆɹÅ×ŌÖĪĒųÖŠæ¼ÕęĢā ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com