
(9��)����A ~ F�dz��л�ѧ�е�����ʵ�飬�밴Ҫ����գ�

(1)Cʵ���н����Ŀ���� ��
(2)Bʵ���к���ȼ�յĻ�ѧ����ʽΪ ��ʵ��˵�����������Լռ������ ��ʵ��ɹ��Ĺؼ��� (�����)��
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
(3)Eʵ�� �ȵ�����������Һ����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ �ٵ�����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ
(4)����ʵ�����ܴﵽʵ��Ŀ������ȷ���� (����ĸ)��
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)����A ~ F�dz��л�ѧ�е�����ʵ�飬�밴Ҫ����գ�
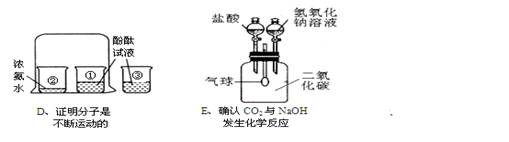
(1)Cʵ���н����Ŀ���� ��
(2)Bʵ���к���ȼ�յĻ�ѧ����ʽΪ ��ʵ��˵�����������Լռ������ ��ʵ��ɹ��Ĺؼ��� (�����)��
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
(3)Eʵ�� �ȵ�����������Һ����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ �ٵ�����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ
(4)����ʵ�����ܴﵽʵ��Ŀ������ȷ���� (����ĸ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)����A ~ F�dz��л�ѧ�е�����ʵ�飬�밴Ҫ����գ�

(1)Cʵ���н����Ŀ���� ��
(2)Bʵ���к���ȼ�յĻ�ѧ����ʽΪ ��ʵ��˵�����������Լռ������ ��ʵ��ɹ��Ĺؼ��� (�����)��
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
(3)Eʵ�� �ȵ�����������Һ����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ �ٵ�����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ
(4)����ʵ�����ܴﵽʵ��Ŀ������ȷ���� (����ĸ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ���б�ҵ��ѧҵ���濼�Ի�ѧ�Ծ����������� ���ͣ��ƶ���
��9�֣�AΪ�������ʣ�B��C��DΪ���ֲ�ͬ���Ļ�������ʼ����Ӧ�Ĺ�ϵ����ͼ��ʾ��ͼ�С���������ʾ���������������ܷ�����Ӧ��. �ش���������. 
��1��A�� B��_____ __ C��___ ____ D��____ ___
��2��д��A��B��Ӧ�Ļ�ѧ����ʽ�� .
��3��д��C��D��Ӧ�Ļ�ѧ����ʽ�� ���������Ӧ������ .
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ��̽����
(9��)����A ~ F�dz��л�ѧ�е�����ʵ�飬�밴Ҫ����գ�

(1)Cʵ���н����Ŀ���� ��
(2)Bʵ���к���ȼ�յĻ�ѧ����ʽΪ ��ʵ��˵�����������Լռ������ ��ʵ��ɹ��Ĺؼ��� (�����)��
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
(3)Eʵ�� �ȵ�����������Һ����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ �ٵ�����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ
(4)����ʵ�����ܴﵽʵ��Ŀ������ȷ���� (����ĸ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com