



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
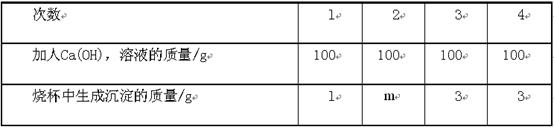
| ���� | 1 | 2 | 3 | 4 |
| ����Ca��OH��2��Һ������/g | 100 | 100 | 100 | 100 |
| �ձ������ɳ���������/g | 1 | m | 3 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����о�У���꼶��һ��ģ��������ѧ�Ծ��������棩 ���ͣ�������
��5�֣�ʵ��������һƿ���ʱ�������NaOHҩƷ������һ������ת��ΪNa2CO3
|
|
|
|
���� |
1 |
2 |
3 |
4 |
|
����Ca(OH)2��Һ������/�� |
100 |
100 |
100 |
100 |
|
�ձ������ɳ���������/�� |
1 |
m |
3 |
3 |
��1��m=����������
��2����10�˸�ҩƷNa2CO3�е�����
��3�������μ���Ca(OH)2��Һ��ַ�Ӧ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ����ϳ��ݾ�����ѧ ���ͣ�������
(6��)ʵ��������һƿ���ʱ�������NaOHҩƷ������һ������ת��ΪNa2C03��ȡlOg��ҩƷ�����ձ��У���154gˮ�����Һ����400gһ����������������Ca(0H)2��Һ���Ĵμ��˸��ձ��У���ַ�Ӧ������ɳ������������ݼ�¼���±���

(1)m=____g,
(2)��10g��ҩƷ��Na2C03������,
(3)�����μ���Ca(OH)2��Һ��ַ�Ӧ��������Һ���������������Ƕ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | 1 | 2 | 3 | 4 |
| ����Ca��OH��2��Һ������/g | 100 | 100 | 100 | 100 |
| �ձ������ɳ���������/g | 1 | m | 3 | 3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com