
����Ŀ��ʵ������һ�ݲ��ֱ�����������þ��þ����Ʒ��δ֪Ũ�������40%������������Һ����ȤС��ͬѧΪ������Ʒ�н���þ���������������ͼ��������������������������̽����
��1���������ܽ���Ʒ����Ʒ��Ͼ��ȣ����ⶨ��������������ʵ�����������ʾ��
ʵ����� | ��ȡ��Ʒ������g�� | ��������������g�� | ��������������g�� |
�� | 16.0 | 60.0 | 0.5 |
�� | 16.0 | 130.0 | 1.0 |
�� | 16.0 | 150.0 | 1.0 |
þ����Ʒ�н���þ�������ٷֺ���Ϊ ��
��2����ʵ�������Һ�м���40%������������Һ�����ɳ������������������������Һ�����ı仯��ϵ��ͼ��ʾ�����������������������д��������̣� 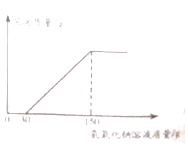
���𰸡�
��1��75.0%
��2���⣺MgO+2HCl=MgCl2+H2O��Mg+2HCl=MgCl2+H2����MgCl2+2NaOH=Mg��OH��2��+2NaCl��HCl+NaOH=NaCl+H2O���ɵù�ϵʽ��HCl��NaOH����150.0gϡ�������Ȼ��������Ϊy
HCl�� | NaOH |
36.5 | 40 |
y | 150g��40% |
![]()
y=54.75g
�������������Ϊ ![]() =36.5%
=36.5%
���������������Ϊ36.5%
���������⣺��1���Աȵڢ����з��֣��Աȵڢ�������������������130g���ӵ�150gʱ������������û�����ӣ�˵���ڢ�����þ��ȫ��Ӧ��
����Ʒ�н���þ������Ϊx��
Mg+2HCl=MgCl2+ | H2�� |
24 | 2 |
x | 1.0g |
![]()
x=12.0g
��Ʒ�н���þ�������ٷֺ���Ϊ�� ![]() =75.0%��
=75.0%��
�𣺣�1����Ʒ�н���þ�������ٷֺ���Ϊ75.0%����2���������������Ϊ36.5%��
�����㾫����������Ҫ�����˸��ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�㣬��Ҫ���ո����ʼ�������=ϵ������Է�������֮�Ȳ�����ȷ�����⣮


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д� С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ȵ�a��b���������,����a��Ϊ���������,��b���л����������,�ֱ�ͬʱ����,���зų���������(m)�뷴Ӧʱ��(t)�Ĺ�ϵͼ������ȷ���ǣ� ��
A.
B.
C.
D.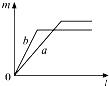
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƽ�������ϸ���һֻ������ͬ���ձ����ֱ�������������������������������ϡ���ᣬ����ƽ������ƽ�⣮Ȼ�������̵��ձ��м���4.0����Ԫ�ص���������Ϊ50%��̼����������ƵĹ������ʹ֮��ȫ��Ӧ����ʹ��ƽ�ָ�ƽ�⣬�������̵��ձ��м��루 ��
A.3.5g����������Һ
B.2.8g������
C.2.8g̼������Һ
D.2.4gþ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧΪ�˲ⶨͭþ�Ͻ���Ʒ��ͭ��������������100gϡ�����2�μ��뵽ʢ��5g����Ʒ���ձ��У��������������������˵���д�����ǣ� ��
���� | ʵ��ǰ | ��1�� | ��2�� |
����ϡ���������/g | 0 | 50 | 50 |
ʣ����������/g | 5 | 3 | 2 |
A.��1�μ���ϡ�����ʣ������л���þ
B.��2���������ϡ����δ��Ӧ��
C.ÿ50gϡ�����1gþǡ����ȫ��Ӧ
D.�Ͻ���Ʒ��ͭ����������Ϊ40%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����¼������ʣ������� ��Һ�� ������������ �ܸɱ� �ݸ������ �������� ������ͭ��Һ ��ϡ������ ���ˮ��������ţ����ڴ��������_____�����ڻ�������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩƷ��ȡ��ԭ��
��1����������______ҩƷ
��2����Ҫ�ѱǿ״յ�______��ҩƷ����ζ
��3������______�κ�ҩƷ��ζ��
��4��û��˵������Һ��һ��ȡ______������ֻ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й��й����ĺ���ӵ�к���ĸ��������������Ѳ������й���ңԶ�����룮���캽ĸ��Ҫ�����Ľ����ѣ�������Ӳ�ȴ��۵�ߡ�������������ʴ�����������ܣ�����Ϊ��δ��������������������ȡ�����ѵ���Ҫ���չ������£������й�����������ǣ� �� 
A.TiCl4�ڸ�����������Mg��Ӧ���ɽ���Ti�����û���Ӧ
B.TiCl4���ڻ����������������
C.�������������еõ��Ľ������л��������������ʣ��ɼ�����ϡ�����ȥ
D.�������������ȡ���أ�����Ϊ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019�������绷�������й�ȷ���������������챣��ս�������ж�����������������֮��Υ������
A.����ֲ���������B.��ũ���в����ĽոѾ͵ط���
C.������ˮ����D.��ҵ��ˮ����ŷ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com