
 »ÆѧÓėÉś»īĆÜĒŠĻą¹Ų
»ÆѧÓėÉś»īĆÜĒŠĻą¹Ų·ÖĪö ĪļÖŹµÄŠŌÖŹ¾ö¶ØĪļÖŹµÄÓĆĶ¾£¬øł¾ŻŅŃÓŠµÄĪļÖŹµÄŠŌÖŹ½ųŠŠ·ÖĪö½ā“š¼“æÉ£®øł¾Ż±źĒ©ŠÅĻ¢½įŗĻ»ÆѧŹ½¼ĘĖć£¬øł¾ŻČÜŅŗĻ”ŹĶĒ°ŗóČÜÖŹÖŹĮæ²»±ä½ā“š£¬øł¾Ż»ÆѧÓėÄÜŌ“µÄÖŖŹ¶½ā“š¼“æÉ£®
½ā“š ½ā£ŗ£Ø1£©A£®øɱł B£®ĢĒĄą C£®Ź³ŃĪ D£®ŃĪĖį
¢ŁÓĆÓŚŹ³Ę·µ÷Ī¶µÄŹĒŹ³ŃĪ£¬¹ŹĢī£ŗC£»
¢ŚĪøŅŗÖŠµÄŃĪĖįæÉŅŌ°ļÖśČĖĢåĻū»Æ£¬¹ŹĢī£ŗD£»
¢ŪŃĪĖįÄÜÓė½šŹōŃõ»ÆĪļ·“Ó¦£¬æÉŅŌÓĆÓŚ½šŹō³żŠā£¬¹ŹĢī£ŗD£»
¢ÜøɱłÉż»ŖĪüČČ£¬æÉÓĆ×÷ČĖ¹¤½µÓź£¬¹ŹĢī£ŗA£®
£Ø2£©¢ŁŅ»ŗŠÅ£ÄĢÖŠÖĮÉŁŗ¬ÓŠ$\frac{250ml}{100ml}”Į3.3g$=8.25g Ö¬·¾£¬¹ŹĢī£ŗ8.25£»
¢ŚōĒ»łĮ×ĖįøĘÖŠøĘÓėŃõµÄÖŹĮæ±ČĪŖ£ŗ£Ø40”Į10£©£ŗ£Ø16”Į26£©=25£ŗ26£»ĒąÉŁÄźČ±øĘ»įµ¼ÖĀŲžŁĶ²”£¬¹ŹĢī£ŗ25£ŗ26£¬ŲžŁĶ²”£®
¢ŪĆæŗŠÅ£ÄĢŗ¬ÓŠøĘŌŖĖŲµÄÖŹĮæĪŖ£ŗ$\frac{250ml}{100ml}”Į0.11g=0.275g$£¬“ÓÅ£ÄĢĪüŹÕµÄøĘŌŖĖŲµÄÖŹĮæĪŖ£ŗ0.6g”Į90%=0.54g£¬¹ŹŠčŅŖÅ£ÄĢ£ŗ$\frac{0.54g}{0.275g/ŗŠ}$”Ö2ŗŠ£¬¹ŹĢī£ŗ2£»
£Ø3£©³“²ĖŹ±£¬Č¼ĘųŌīµÄ»šŃę³Ź»ĘÉ«£¬¹ųµ×³öĻÖŗŚÉ«ĪļÖŹ£¬ĖµĆ÷ŃõĘųµÄĮæ²»×ć£¬“ĖŹ±æɽ«Ōī¾ßµÄ½ų·ēæŚµ÷“󣬹ŹĢī£ŗµ÷“ó£»
£Ø4£©40%µÄĻ”ĮņĖįČÜŅŗÖŠČÜÖŹÓėČܼĮµÄÖŹĮæ±ČĪŖ40%£ŗ60%=2£ŗ3£¬300g ÕāŃłµÄĻ”ĮņĖįÖŠŗ¬ÓŠĮņĖįµÄÖŹĮæĪŖ£ŗ300g”Į40%=120g£¬ŠčŅŖÅØĮņĖįµÄÖŹĮæĪŖ£ŗ120g”Ā98%=122.4g£¬ŌņŠčŅŖĖ®µÄÖŹĮæĪŖ£ŗ300g-122.4g=177.6g£¬¹ŹŠčŅŖĖ®µÄĢå»żĪŖ£ŗ177.6g”Ā1g/mL=177.6mL£¬ÅØĮņĖįµÄĻ”ŹĶ²»ŅŖÓĆĶŠÅĢĢģĘ½£¬¹ŹĢī£ŗ2£ŗ3£¬177.6£¬A£»
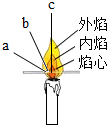
£Ø5£©³£¼ūµÄ»ÆŹÆČ¼ĮĻÓŠĆŗ”¢ŹÆÓĶŗĶĢģČ»ĘųµČ£®ĢģČ»ĘųŌŚæÕĘųÖŠĶźČ«Č¼ÉÕÉś³É¶žŃõ»ÆĢ¼ŗĶĖ®£®ĘūÓĶĶźČ«Č¼ÉյIJśĪļÖŠ¶ŌĘųŗņ±ä»ÆÓŠÓ°ĻģµÄŹĒ¶žŃõ»ÆĢ¼£¬¹ŹĢī£ŗŹÆÓĶ£¬CH4+2O2$\frac{\underline{\;µćČ¼\;}}{\;}$CO2+2H2O£¬¶žŃõ»ÆĢ¼£®
µćĘĄ ±¾Ģāæ¼²éµÄŹĒ»ÆѧÓėÉś»īµÄÖŖŹ¶£¬ÕĘĪÕ³£¼ūµÄĪļÖŹŠŌÖŹŗĶÓĆĶ¾µÄ¹ŲĻµŹĒÕżČ·½ā“š±¾ĢāµÄ¹Ų¼ü£®


 »ĘøŌŠ”דŌŖĶ¬²½¼ĘĖćĢģĢģĮ·ĻµĮŠ“š°ø
»ĘøŌŠ”דŌŖĶ¬²½¼ĘĖćĢģĢģĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĢģČ»Ė®ŗĶ“æ¾»Ė®Ć»ÓŠŹ²Ć“Ēų±š | |
| B£® | ¹żĀĖŗóµÄĢģČ»Ė®¾ĶŹĒÕōĮóĖ® | |
| C£® | Ó²Ė®ŗĶČķĖ®µÄĒų±šŌŚÓŚĖ®ÖŠÓŠĆ»ÓŠČÜÓŠøĘ”¢Ć¾»ÆŗĻĪļ | |
| D£® | Éč·Ø³żČ„Ó²Ė®ÖŠµÄøĘ”¢Ć¾»ÆŗĻĪļ£®æÉŅŌŹ¹Ó²Ė®Čķ»Æ³ÉČķĖ®£® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
 »ÆѧŹĒŅŌŹµŃéĪŖ»ł“”µÄŅ»ĆÅѧæĘ£¬Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĻĀŹµŃéĢ½¾æ£ŗ¶ŌĄÆÖņ£ØÖ÷ŅŖ³É·ÖŹĒŹÆĄÆ£©¼°ĘäČ¼ÉÕ½ųŠŠĮĖČēĻĀĢ½¾æ£®
»ÆѧŹĒŅŌŹµŃéĪŖ»ł“”µÄŅ»ĆÅѧæĘ£¬Ä³Š£»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠĮĖČēĻĀŹµŃéĢ½¾æ£ŗ¶ŌĄÆÖņ£ØÖ÷ŅŖ³É·ÖŹĒŹÆĄÆ£©¼°ĘäČ¼ÉÕ½ųŠŠĮĖČēĻĀĢ½¾æ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
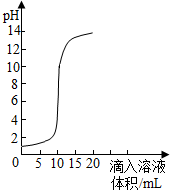 ŹŅĪĀŹ±£¬½«ĒāŃõ»ÆÄĘČÜŅŗÓėŃĪĖįÓƵĪ¼Ó·½Ź½·“Ó¦Ź±£¬ČÜŅŗµÄpHĖęµĪČėČÜŅŗĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠÓŠ¹ŲĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
ŹŅĪĀŹ±£¬½«ĒāŃõ»ÆÄĘČÜŅŗÓėŃĪĖįÓƵĪ¼Ó·½Ź½·“Ó¦Ź±£¬ČÜŅŗµÄpHĖęµĪČėČÜŅŗĢå»ż±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠÓŠ¹ŲĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | øĆĶ¼Ļó±ķŹ¾µÄŹĒ½«ĒāŃõ»ÆÄĘČÜŅŗµĪČėŃĪĖįÖŠ | |
| B£® | ĒāŃõ»ÆÄĘČÜŅŗŗĶŃĪĖįĒ”ŗĆĶźČ«·“Ó¦Ź±£®ČÜŅŗµÄpHµČÓŚ7 | |
| C£® | µĪČėČÜŅŗĢå»żĪŖ15mLŹ±£¬ŌŁ¼Ó¼øµĪ×ĻÉ«ŹÆČļŹŌŅŗČÜŅŗ³ŹĄ¶É« | |
| D£® | µ±µĪČėČÜŅŗµÄĢå»żĪŖ5mLŹ±£¬ĖłµĆČÜŅŗÖŠµÄČÜÖŹÖ»ÓŠNaCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
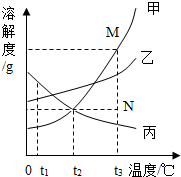 ČēĶ¼ŹĒ¼×”¢ŅŅ”¢±ūČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ¬ĒŅĖüĆĒČÜÓŚĖ®Ź±ČÜŅŗĪĀ¶Č¾łĪŽĆ÷ĻŌ±ä»Æ£®»Ų“šĻĀĮŠĪŹĢā
ČēĶ¼ŹĒ¼×”¢ŅŅ”¢±ūČżÖÖ¹ĢĢåĪļÖŹµÄČܽā¶ČĒśĻߣ¬ĒŅĖüĆĒČÜÓŚĖ®Ź±ČÜŅŗĪĀ¶Č¾łĪŽĆ÷ĻŌ±ä»Æ£®»Ų“šĻĀĮŠĪŹĢā²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ö÷ŅŖŌĄķĪŖ£ŗĄūÓĆ½¹ĢæµÄ»¹ŌŠŌÖĆ»»³öĢśæóŹÆÖŠµÄĢś | |
| B£® | Ö÷ŅŖŌĮĻĪŖĢśæóŹÆ”¢½¹ĢæŗĶŹÆ»ŅŹÆµČ | |
| C£® | Ö÷ŅŖÉč±øĪŖøßĀÆ | |
| D£® | Ö÷ŅŖ²śĪļĪŖÉśĢś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĀČ»ÆÄʵÄČܽā¶ČŹÜĪĀ¶Č±ä»ÆµÄÓ°ĻģŗÜŠ” | |
| B£® | ŌŚ”°Ņ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĀČ»ÆÄĘČÜŅŗµÄÅäÖĘ”±µÄŹµŃéÖŠ£¬Ź¹ÓĆ²£Į§°ō½Į°č£¬¼ÓæģĀČ»ÆÄʵÄČܽā | |
| C£® | ĀČ»ÆÄʵÄĖ®ČÜŅŗÄܵ¼µēŹĒŅņĪŖĀČ»ÆÄĘŌŚĖ®ÖŠ»į½āĄė³öÄÜ×ŌÓÉŅĘ¶ÆµÄNa+ŗĶCl- | |
| D£® | ŌŚ”°“ÖŃĪÖŠÄŃČÜŠŌŌÓÖŹµÄČ„³ż”±µÄŹµŃéÖŠ£¬µ±Õō·¢ĆóÖŠµÄĀĖŅŗČ«²æÕōøÉŗó²ÅÄÜĶ£Ö¹¼ÓČČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Cl-”¢NO3-”¢K+”¢Na+ | B£® | SO42-”¢NO3-”¢K+”¢OH- | ||
| C£® | CO32-”¢SO42-”¢Mg2+ | D£® | NH4+”¢Cl-”¢OH-”¢K+ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com