
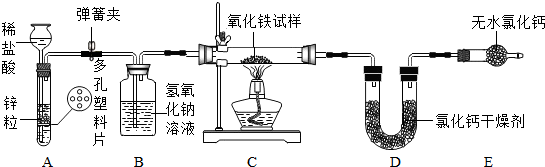
·ÖĪö øł¾ŻĒāĘųµÄÖĘČ”ŗĶŠŌÖŹ”¢ŃĪĖįµÄŠŌÖŹ”¢ĘųĢåµÄøÉŌļ”¢·“Ó¦µÄŌĄķ”¢ŹµŃé·½°øÉč¼ĘÓėĘĄ¼ŪµČÖŖŹ¶£¬½ųŠŠ×ŪŗĻ·ÖĪö”¢ĶʶĻŗĶ½ā“š¼“æÉ
½ā“š ½ā£ŗ£Ø1£©A·¢Éś×°ÖĆÓėĘōĘÕ·¢ÉśĘ÷µÄŌĄķĻąĶ¬£¬ĘäÓŵćĪŖ£ŗÄÜæŲÖĘ·“Ó¦µÄ·¢ÉśŗĶÖÕÖ¹£»¹ŹĢī£ŗÄÜæŲÖĘ·“Ó¦µÄ·¢ÉśŗĶÖÕÖ¹£»
£Ø2£©ĒāĘųŗĶŃõ»ÆĢś¼ÓČČ·“Ӧɜ³ÉĢśŗĶĖ®£¬¹ŹĢī£ŗ3H2+Fe2O3$\frac{\underline{\;\;”÷\;\;}}{\;}$2Fe+3H2O£»
£Ø3£©Éś³ÉĖ®µÄÖŹĮæĪŖ£ŗ26.1g-23.4g=2.7g£¬ÉčŃõ»ÆĢśµÄÖŹĮæĪŖx
3H2+Fe2O3$\frac{\underline{\;\;”÷\;\;}}{\;}$2Fe+3H2O
160 54
x 2.7g
$\frac{160}{54}=\frac{x}{2.7g}$ x=8g
¹ŹŃõ»ÆĢśµÄÖŹĮæ·ÖŹżĪŖ£ŗ$\frac{8g}{10g}”Į100%$=80%
¹ŹĢī£ŗ80%£»
£Ø4£©ĪŖĮĖ²»ČĆ“Ó×°ÖĆBÖŠµ¼³öµÄĒāĘų»į“ų³öµÄĖ®ÕōĘų£¬±»×°ÖĆDĖłĪüŹÕ¶ųŅżĘšĪó²ī£»æÉŅŌŌŚ×°ÖĆB”¢CÖ®¼ä£¬ŌöĢķŅ»øöĘųĢåøÉŌļ×°ÖĆ£Ø¼“Ź¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ棩£¬ŅŌ±ćĪüŹÕ“Ó×°ÖĆBÖŠµ¼³öµÄĒāĘų»į“ų³öµÄĖ®ÕōĘų£¬±ÜĆāŌģ³ÉĪó²ī£®¹ŹĢī£ŗæÉŅŌŌŚ×°ÖĆB”¢CÖ®¼ä£¬ŌöĢķŅ»øöĘųĢåøÉŌļ×°ÖĆ£Ø¼“Ź¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ棩£¬ŅŌ±ćĪüŹÕ“Ó×°ÖĆBÖŠµ¼³öµÄĒāĘų»į“ų³öµÄĖ®ÕōĘų£¬±ÜĆāŌģ³ÉĪó²ī£®
µćĘĄ ±¾Ģāæ¼²éµÄÖŖŹ¶µć½Ļ¶ą£¬ÖŖŹ¶Ćę½Ļ¹ć£»ŹĒŅ»µĄ±Č½Ļø“ŌÓµÄ×ŪŗĻŹµŃé·ÖĪöĢā£®½ā“šŹ±£¬Ņ»¶ØŅŖĻøŠÄ”¢ČĻÕęµŲ½ųŠŠ·ÖĪöĢ½¾æ£¬²¢½ųŠŠæĘѧŗĻĄķµÄĶʶĻŗĶÉč¼Ę”¢ĘĄ¼ŪŹµŃé·½°øµČ£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ČēĶ¼ŹĒijŹŌ¼ĮĘæ±źĒ©µÄ²æ·ÖÄŚČŻ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ČēĶ¼ŹĒijŹŌ¼ĮĘæ±źĒ©µÄ²æ·ÖÄŚČŻ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ė¤ĖéĮĖµÄ“ÉĶėŗÜÄŃĘ“ŗĻŌŚŅ»Ęš--·Ö×Ó¼ä“ęŌŚ³āĮ¦ | |
| B£® | ÅŹĒÅŗĢĄČȵď±ŗņĻćĘųĖÄŅē--ĪĀ¶ČŌ½øߣ¬·Ö×ÓµÄŌĖ¶ÆŌ½¾ēĮŅ | |
| C£® | Ē½ÄŚæŖ»ØĒ½ĶāĻć--·Ö×ÓŌŚ²»Ķ£µŲ×öĪŽ¹ęŌņŌĖ¶Æ | |
| D£® | ŗ£±ßÖēŅ¹ĪĀ²ī±ä»Æ±ČɳĮ֊Š”--Ė®µÄ±ČČČČŻ±ČɳŹÆµÄ±ČČČČŻ“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«ÅØĮņĖįĀżĀż×¢ČėŹ¢ÓŠĖ®µÄĮæĶ²Ąļ | |
| B£® | ĒāĘų»¹ŌŃõ»ÆĶŹ±£¬ĻČ¼ÓČČŃõ»ÆĶŗóĶØĒāĘų | |
| C£® | ŹµŃéŹŅÖʱøĘųĢåŹ±£¬ĻČ×°ČėŅ©Ę·£¬ŌŁ¼ģŃé×°ÖƵÄĘųĆÜŠŌ | |
| D£® | ÖĘ×÷¹żĀĖĘ÷Ź±£¬ĀĖÖ½µÄ±ßŌµÓ¦±ČĀ©¶·æŚÉŌµĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé²½Öč | ½āŹĶ»ņ½įĀŪ |
| £Ø1£©½«ĒåŠĀ½ą¾»µÄæÕĘųĶعżĒāŃõ»ÆÄĘČÜŅŗ£¬ŌŁĶعżÅØĮņĖį | ĶعżĒāŃõ»ÆÄĘČÜŅŗÄæµÄŹĒĪŖĮĖæÕĘųÖŠµÄ¶žŃõ»ÆĢ¼£®ĶعżÅØĮņĖįµÄÄæµÄŹĒĪŖĮĖ³żČ„æÕĘųÖŠµÄĖ®ÕōĘų£® |
| £Ø2£©½«ĘųĢåĶعż×ĘČȵÄĶ·Ū | ³żČ„æÕĘųÖŠµÄŃõĘų£® |
| £Ø3£©ŹÕ¼ÆĘųĢ壬²¢²ā¶ØøĆĘųĢåµÄĆÜ¶Č | ĆܶČĪŖ1.2572g/Éż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ĪŅŹŠÄ³Ę·ÅĘæóČŖĖ®£¬ĘäĶā°ü×°ÉĻ²æ·ÖĪÄ×ÖĖµĆ÷ČēĶ¼ĖłŹ¾£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ĪŅŹŠÄ³Ę·ÅĘæóČŖĖ®£¬ĘäĶā°ü×°ÉĻ²æ·ÖĪÄ×ÖĖµĆ÷ČēĶ¼ĖłŹ¾£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ĘųĢå | æÕĘų | CH4 | CO2 | SO2 | H2 | NH3 |
| ±ź×¼×“æöĻĀµÄĆÜ¶Č | 1.29 | 0.72 | 1.98 | 2.86 | 0.09 | 0.77 |
| ±ź×¼×“æöĻĀ1Ģå»żĖ®ÖŠÄÜČܽāĘųĢåµÄĢå»ż | 0.033 | 0.83 | 40 | 0.018 | 680 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
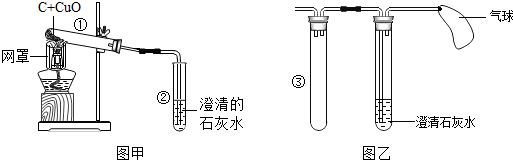
| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ȔɣĮæ·“Ó¦ŗóµÄŹŌ¹Ü¢ŁÖŠµÄ¹ĢĢåÓŚ½ą¾»ŹŌ¹ÜÄŚ£¬µĪČėĻ”ĮņĖįĻ”ĮņĖį Ī¢ČČ£® | A | øĆŗŚÉ«¹ĢĢåŹĒľĢæ |
| B | øĆŗŚÉ«¹ĢĢåŹĒŃõ»ÆĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
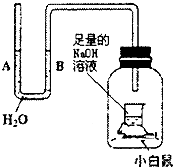 ČēĶ¼ŹĒijŠĖȤŠ”×éÉč¼ĘµÄŅ»Ģ׏µŃé×°ÖĆ£¬×°ÖĆ×ćŅŌĪ¬³ÖŹµŃé¹ż³ĢÖŠŠ”°×ŹóµÄÉśĆü»ī¶Æ£¬ĘææŚĆÜ·ā£®×°ÖĆĘųĆÜŠŌĮ¼ŗĆ£®¾ŹżŠ”Ź±ŗó£¬uŠĪ¹ÜA£®BĮ½“¦µÄŅŗĆę»į³öĻÖĻĀĮŠÄÄÖÖĒéæö£æ£ØNaOHČÜŅŗæÉĪüŹÕ¶žŃõ»ÆĢ¼£©£Ø””””£©
ČēĶ¼ŹĒijŠĖȤŠ”×éÉč¼ĘµÄŅ»Ģ׏µŃé×°ÖĆ£¬×°ÖĆ×ćŅŌĪ¬³ÖŹµŃé¹ż³ĢÖŠŠ”°×ŹóµÄÉśĆü»ī¶Æ£¬ĘææŚĆÜ·ā£®×°ÖĆĘųĆÜŠŌĮ¼ŗĆ£®¾ŹżŠ”Ź±ŗó£¬uŠĪ¹ÜA£®BĮ½“¦µÄŅŗĆę»į³öĻÖĻĀĮŠÄÄÖÖĒéæö£æ£ØNaOHČÜŅŗæÉĪüŹÕ¶žŃõ»ÆĢ¼£©£Ø””””£©| A£® | A“¦ÉĻÉż£¬B“¦ĻĀ½µ | B£® | A”¢BĮ½“¦¶¼ĻĀ½µ | C£® | A“¦ĻĀ½µ£¬B“¦ÉĻÉż | D£® | A”¢BĮ½“¦¶¼²»±ä |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com