

| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� | |
| �� | 50.0g | 1% | 0.1g | 9mL |
| �� | 50.0g | 2% | 0.1g | 16mL |
| �� | 50.0g | 4% | 0.1g | 31mL |
 2KCl+3O2����
2KCl+3O2����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |

| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
| �� | 50.0g | 1% | 0.1g | 9mL |
| �� | 50.0g | 2% | 0.1g | 16mL |
| �� | 50.0g | 4% | 0.1g | 31mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ȡ������ʢ�ж�����̼�ļ���ƿ�� | �а�ɫ���� �а�ɫ���� |
������ȷ ��صĻ�ѧ����ʽ CO2+Ba��OH��2�TBaCO3��+H2O CO2+Ba��OH��2�TBaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����
ij��ȤС��ͬѧ��ʵ�����Ʊ������������������������ʽ�������̽����
| ||
| �� |
| ||
| �� |
| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2��� | |
| �� | 50.0g | 1% | 0.1g | 9mL |
| �� | 50.0g | 2% | 0.1g | 16mL |
| �� | 50.0g | 4% | 0.1g | 31mL |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ���� | ���� | ���� |
| �� |  |
����Ƭ����ˮ����� ����Ƭ����ˮ����� |
�����к���ˮ |
| �� |  |
�ܿ�۲쵽��Һ�� �� �� ɫ��Ȼ��۲쵽�����ݲ��� |
�������Fe2O3 |
| �� | �ռ�������Թ��в��������壬 ��ȼ ��ȼ |
����� | ������� �� �� |
| �� | ����ڳ�ַ�Ӧ���Թ��е����ʵ�����ֽ�� | ��ֽ���к�ɫ���� | ��ɫ�������Ҫ�ɷ��� ̼ ̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
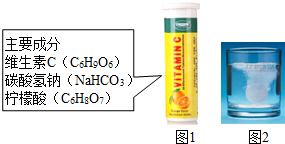 άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����
άC����Ƭ����Ҫ�ɷּ�ͼ1������ˮ�����������ݲ�������ͼ2����ij��ȤС��ͬѧ������ijɷֽ�������̽����| ʵ���� | ʵ����� | ʵ������ |
| �� | ������ͨ������ʯ��ˮ�� | |
| �� | �������ǵ�ľ������������� | �����ǵ�ľ��û�и�ȼ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com