
�����ǵ���Ҫ�ɷ���̼��ƣ�ij��ȤС��Ϊ�˲ⶨ��������CaCO3�ĺ�������ȡ15g�����ǣ����飬�����ձ��У�Ȼ�������м���80gijŨ�ȵ�ϡ���ᣬʹ֮��ַ�Ӧ���������г�CaCO3��������ɷֶ�������ˮ���Ҳ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ��������е���Ӧ���е�B��ʱ����������պ������˼�������һ�룬�Լ��㣨����������1λС����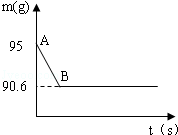
��1������CO2������Ϊ g��
��2���ü�������CaCO3������������
��3������ϡ���������ʵ�����������
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��4�֣��ҹ�Լ���ϱ���ʱ�Ϳ�ʼ���ƻ�ͭ����ͭ��ͭ��п�ĺϽ�������������������������������Ʒ��Ϊ�˲ⶨij��ͭ��ͭ������������ȡ10g��ͭ���뵽50gϡ�����У�ǡ����ȫ��Ӧ����������0.1g������
��1���û�ͭ��Ʒ��ͭ������������
��2��ԭϡ������Һ�����ʵ�����������
����ܰ��ʾ�����ʱ��Ҫ�б�Ҫ������˵���ͼ��㲽��ȣ�ֻд����������֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�Ϊ�˲ⶨij��Ʒ��̼�ᱵ��������������������ʵ�顣ȡһ��������Ʒ��400g������������Ϊ10%��ϡ��������ձ��С��ڻ�ѧ��Ӧ�����ж��ձ������е�ʣ�����������γ�������¼���±��������跴Ӧ���ٽ��У����ʲ�����ˮ��Ҳ����ϡ���ᷴӦ���ձ�������Ϊ25��4g��
| ��Ӧʱ�� | t1 | t2 | t3 | t4 | t5 |
| �ձ���ҩƷ����/g | 516��6 | 507��8 | m | 503��4 | 503��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27��5 g������Ʒ������Ũ�ռ���Һ���ȣ������İ�����100��0g���������ա�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ�����漰�ķ�ӦΪ��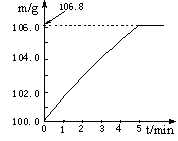
(NH4)2SO4+2NaOH Na2SO4+2H2O+2NH3���� 2NH3+H2SO4=(NH4)2SO4��
Na2SO4+2H2O+2NH3���� 2NH3+H2SO4=(NH4)2SO4��
����㣺
��1����ȫ��Ӧ��������� g��
��2���û��ʵĺ�����Ϊ ����ȷ��0��1%���������ֻ������� (����ϸ��ϸ��ϸ�����狀�����Ϊ20%����)��Ʒ��
��3�����������������������������д��������̣���
��4����ʵ������а�������ȫ���գ�����ʵ��������炙��ʵĺ���������ʵ��ֵ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(6��)Ϊ�ⶨCuһZn�Ͻ����ɣ�С��ͬѧ���øúϽ��ĩ��ϡ���ᷴӦ������������ʵ�飬�������ʵ�����ݼ�¼���£�
| | ��һ�� | �ڶ��� | ������ |
| ��ȡ�Ͻ������/g | 1O | 10 | 20 |
| �������������/g | 50 | 80 | 50 |
| ��������������/g | 0��2 | 0��2 | 0��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
����ơ���һ�ֿ�����ϸ�����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȡ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���
��1��̼����и�Ԫ�ص�����������____%��ʳ��������̼��������ڷ�ֹ����ȱ�������_______________________���� ����������֢����ƶѪ��������
��2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����_____________���������Ӧ���ͣ���
��3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽 ��֤�������к���ʯ��ʯ��
��4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ��������ơ���ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ȤС��Ϊ̽������������ͭ�ۡ��������ͭ��������������ȡһ�������Ļ���������ͼʵ��װ�ý���ʵ�飺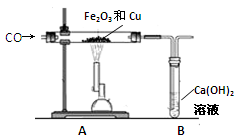
��1��ʵ��ʱҪ����ͨһ����̼���壬����ȡ� ��Ŀ���� ��
��2��װ��A�з�����Ӧ�ķ���ʽΪ ��
��3��ʵ��װ��B�е������� ��������Ӧ�Ļ�ѧ����ʽΪ ��
��4����װ�õ������һ���Բ���֮������ĸĽ������� ��
[��������]
����ȤС�鰴�տ�ѧ�ķ������ʵ��Գ�ַ�Ӧ��Ĺ��ڹ���X�������º���ʵ��̽����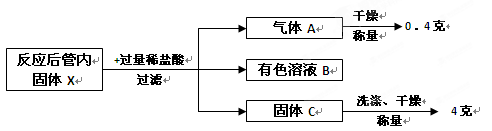
�ش��������⣺
��1��д������X��ϡ���ᷴӦ�ķ���ʽ�� ��
��2����ɫ��ҺB�к��е����ʣ� ���ѧʽ����
��3����������ʵ�����̣�����ԭ������������ͭ�ۡ���ͭ����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��400 t��Fe2O3��������Ϊ80���ij�����ʯ������������̼����������Ϊ3�����������ٶ�?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ������ȡ�����Ļ�ѧ����ʽ��Zn+H2SO4�TZnSO4+H2��������40g��������Ϊ30%��������Һ����ˮϡ����120g��ȡ��ϡ�ͺ����Һ5g���Լ��㣬��5g��Һ������������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com