

| | ���� | ������ |
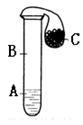
| �� | ��ֹˮ��a����Һ©����������Һ��������Թܺر�ֹˮ��a����Һ©�������� | Һ��ֲ㣬���۳�����ɫС����Һ��ֲ�Ľ����Ϸ������������������ݣ��²���Һ��ɺ�ɫ������ѹ��B�С������Ƶ��������ʣ�д��һ�㼴�ɣ� �� |
| �� | ��ֹˮ��b����B�в�����Һ����C�У�Ѹ�ٹر�ֹˮ��b�� | ��Ӧ�Ļ�ѧ����ʽΪ |
| �� | ��ֹˮ��c�� | ��Һ��ɫ��ʧ�� ��Ӧ�Ļ�ѧ����ʽΪ �� |
| �� | ��ȼ�ŵ�ľ���쵽���ܼ��촦����ֹˮ��a�� �ڻ����Ϸ���һ��������ձ��� | ����ȼ�գ���������ɫ���� �ձ��ڱ�����ɫҺ�����ɣ�A������Ϊ������ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
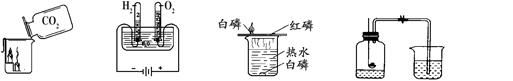
| A��CO2��� | B��ˮ��� | C��̽��ȼ������ | D�����������������IJⶨ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
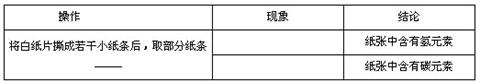
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ죬�γɴ�����ĭ�ӵ��ܳ�� |
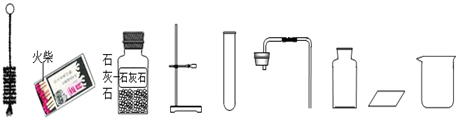
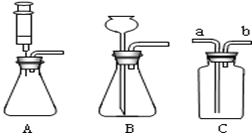
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 Ũ�� Ũ����Ӧ���� | 30% H2O2 | 15% H2O2 | 10% H2O2 | 5% H2O2 |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| ���������� | 360s | 480s | 540s | 720s |
| MnO2������������ | 40s | 60s | 120s | 180s |
| MnO2���������� | 10s | 25s | 60s | 120s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ���� | �۵�u�� | �е�u�� | ȼ��ʱ�¶Ȩu�� |
| ʯ�� | 50��70 | 300��550 | Լ600 |
| �� | 1535 | 2750 | Լ1800 |
| �� | 97.8 | 883 | Լ1400 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com