
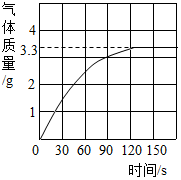
 ˮ������֮Դ������֮���������౦�����Ȼ��Դ������ÿ���˶�Ҫ����ˮ������ˮ����Լ��ˮ��
ˮ������֮Դ������֮���������౦�����Ȼ��Դ������ÿ���˶�Ҫ����ˮ������ˮ����Լ��ˮ������ ��1�����ݹ����ǽ�������ˮ�Ĺ�����Һ������һ�ַ�����𣻣�2����ͼ�п����Կ������۵㴦��Ϊ���ɶ�����̼��������
���ݶ�����̼�����������̼��Ƶ��������ٸ���$\frac{̼��Ƶ�����}{ˮ��������}$��100%�������ˮ����̼��Ƶ���������������ˮ����������̼��Ƶ��������������þ���������ٸ���������þ�����������������þ��Ӧ����������������ݶ�����̼�����������̼��Ʒ�Ӧ�����������������������þ��Ӧ��������������̼��Ʒ�Ӧ�������������������Ϊ���������������
��� �⣺
��1�����С�cָ�ꡱ����ͨ�����˲����ﵽ��
��2����ͼ�пɿ������ɵĶ�����̼�����3.3g��
��̼��Ƶ�����Ϊx����̼��Ʒ�Ӧ��������Һ�����ʵ�����Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 3.3g
$\frac{100}{x}=\frac{73}{y}=\frac{44}{3.3g}$��
x=7.5g��y=5.475g
ˮ����̼��Ƶ���������=$\frac{7.5g}{10.4g}$��100%=72.1%��
��ˮ����̼��Ƶ���������Ϊ72.1%��
��3��������þ������=10.4g-7.5g=2.9g
���������þ��Ӧ��������Һ�����ʵ�����Ϊz��
Mg��OH��2+2HCl=MgCl2+2H2O
58 73
2.9g z
$\frac{58}{2.9g}=\frac{73}{z}$��z=3.65g
����������Һ������Ϊ$\frac{5.475g+3.65g}{10%}$=91.25g
��������Ҫ��������Ϊ10%�������������91.25g��
���� ������ĵڶ�С��Ƚ��ѣ�����������ʱӦ���������ַ���������ǰ��ֱ��������֪������


 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��� | B�� | ���� | C�� | ���� | D�� | ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢܢ٢ݢ� | B�� | �ܢݢۢ٢� | C�� | �٢ݢۢܢ� | D�� | �ܢۢݢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Թ�ȡ���Լ�ƿ�е�Na2CO3��Һ������ȡ�����࣬Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� | |
| B�� | Ba��NO3��2����ˮ���ɽ�����Ba��NO3��2�ķ�Һ����ˮ���У�����ˮ������ˮ�� | |
| C�� | ����������ʹNaCl ����Һ������ʱ��Ӧ����������NaCl ��Һȫ���������� | |
| D�� | ��Ȼ��й©�������رշ��Ų�����ͨ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
| ʵ����� | ���� | ���� | ���� |
| ����ϡ��������/g | 108 | 98 | 98 |
| ����ͭþ�Ͻ���Ʒ����/g | 7.2 | 7.8 | 7.2 |
| ��ַ�Ӧ���ձ���ʣ����������/g | 115.0 | 105.6 | 105.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
| ���� | ��1�� | ��2�� | ��3�� | ��4�� |
| ����ϡ��������/g | 25 | 25 | 25 | 25 |
| ��Ӧ���ձ������ʵ�������/g | 35.2 | 58.4 | 82.6 | 107.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com