
ij���������п�ڷ���Һʹ��˵����IJ����������£�
��1�����������[Ca(C6H11O7)2]�к��� ��Ԫ�أ����и�Ԫ������Ԫ�ص�������Ϊ ��
��2����������Ƶ���Է�������Ϊ ��
��3������������и�Ԫ�ص���������Ϊ ��
��4��ÿƿ�к���Ԫ�ص�����Ϊ mg��
��5��ij��ÿ�շ���һ���������������п�ڷ���Һ������ÿ������Ŀڷ�Һ��15 mg��������п����ͬʱ����ĸ�Ԫ�ص�����Ϊ mg��
��1��4 5:28 ��2��430 ��3��9.3% ��4��55.8 ��5��27.9
�������������
��1���ӻ�ѧʽ���������������[Ca(C6H11O7)2]�к���Ca��C ��H��O ����Ԫ�أ����и�Ԫ������Ԫ�ص�������Ϊ40��1��16��14=5��28��
��2����Է���������ָ��ѧʽ�и�ԭ�ӵ����ԭ������֮�͡���������Ƶ���Է�������Ϊ40��12��12��1��22��16��14=430��
��3������������и�Ԫ�ص���������Ϊ ��100%=9.3%��
��100%=9.3%��
��4��ÿƿ�����������600 mg����ÿƿ�к���Ԫ�ص�����Ϊ600��9.3%=55.8mg��
��5���������⣬�����������п�ڷ���Һ���������������������п��������Ϊ600��30=20��1������ÿ������Ŀڷ�Һ��15 mg��������п����ͬʱ�������������Ƶ�����Ϊ300mg�����к���Ԫ�ص�����Ϊ300 mg��9.3%=27.9mg��
���㣺���ݱ�ǩ���л�ѧʽ�ļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ع����к���һ��ǿ���°������ù�أ�C17H14O6��������ʳ�û��������������䣬��ش�
��1������ù���ӣ�C17H14O6���к��� ��ԭ�ӣ�
��2������ù�أ�C17H14O6����̼��������Ԫ�ص�������Ϊ ��
��3������ù�ط����У�C17H14O6��̼ԭ�ӣ���ԭ�ӣ���ԭ�Ӹ�����Ϊ ��
��4������ù�أ�C17H14O6������Է��������� ��
��5��15.7g����ù���к��� g��Ԫ�ء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����������г��õ�һ���ᣬ�仯ѧʽΪCH3COOH������㣺
��1���������Է���������
��2��������̼Ԫ�ص�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���������ݷ���������ʹ��ķ�������ȷ��ij�߸�Ƭ����ͼ����ʹ��˵���鲿���������£�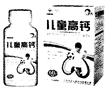
�����ϡ�̼���( CaCO3)����¶�������ǡ����ۡ�ά����D��
�����2��5g/Ƭ��ÿƬ�к���0��5 g��
��ʳ�÷�����ÿ��1�Σ�ÿ��1Ƭ����ʳ��
��1��̼���( CaCO3)����Է�������Ϊ ��
̼����и�Ԫ�ص�������Ϊ ��
��2�����˸߸�Ƭ��������Ԫ�ض�����̼��ƣ��Լ���һƬ�ø߸�Ƭ������̼��Ƶ���������������������̣�
��3����������ƵĻ�ѧʽΪCa( C6 H11O7)2������Է�������Ϊ430��ij��������Ʊ���ƷÿƬ�����������0��5 g��1Ƭ�߸�Ƭ������Ԫ�ص�����Լ�൱��_______Ƭ��������ȡ����������������Ʊ���Ʒ������Ԫ�ص�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ�����ͻ��ȼ�ϻ�ѧʽΪN(NO2)3����
��1��������Է�������Ϊ ��������Ԫ���뵪Ԫ�ص�������Ϊ ��
��2��N(NO2)3�е�Ԫ�ص����������Ƕ��٣�(��ʽ���� )
��3��N(NO2)3�к��е�Ԫ�ص������Ƕ��٣�(��ʽ���� )
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�������Ƴɾ�������Ʒ������Ҫ�ɷֵĻ�ѧʽΪNaAlSi2O6������㣺
��1��NaAlSi2O6����Է�������Ϊ�� ��
��2��NaAlSi2O6�й�Ԫ�غ���Ԫ�ص�������Ϊ�� ��������������ȣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ع����к���һ��ǿ���°������ù��B2��C17H14O6��������ʳ�û��������������䣬��ش�
��1������ù��B2����Է�������Ϊ ��
��2������ù��B2��̼���⡢������Ԫ�ص�ԭ�Ӹ�����Ϊ ��
��3��15.7g����ù��B2�к��� g��Ԫ�أ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ҵ����CaO��HNO3Ϊԭ���Ʊ�Ca(NO3)2?6H2O���壬Ϊȷ���Ʊ������мȲ�����ˮ��Ҳ�����ˮ�����������������������ԼΪ
| A��41��2% | B��53��8% | C��58��3% | D��70��0% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪ij��ӳ����ʾ��ͼ���£�����˵����ȷ���ǣ� ��
| A���÷�Ӧ�����������غ㶨�� | B���μӷ�Ӧ�ķ��Ӹ�������5:2 |
| C���÷�Ӧ�з�Ӧ�ﶼ�ǻ����� | D���÷�Ӧǰ��ķ�������ı� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com