
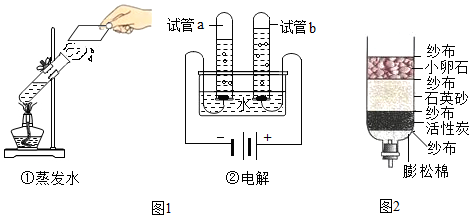
| ʵ��װ�� |  ����������ȼ�� |  �ⶨ�������������� |  ��˿��������ȼ�� |
���� ��1�����ݴ�����Ķ�������������ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�������ǣ�
��2��ˮ�����������������仯����֤�����ӵIJ����˶��������Ӽ������������Ϥ���ˮ��ʵ����̡������������Լ����������������ʣ�����������������Ƶ���Һ�����Խ�ǿ�����������֤�����ķ�����������������е�֪ʶ���з��������ˮʱ�������ɵ������븺�����ɵ��������������1��2��
��3������С��ʯ��ʯӢɳ������������ȥ������ˮ�����ʽ��н�𣻸��ݻ���̿�����������ã���������ˮ�е�ɫ�غ���ζ���н�𣻸��ݿ��÷���ˮ�ж����õ�ˮ��Ӳˮ������ˮ���н��
��4������������ȼ�����ɶ���������������Ⱦ���������ˮ���������ն�������ģ��ⶨ����������������ˮ������ͨ��ˮ����ı仯�ó�������O2�����������˿��������ȼ�գ�����ƿ�м���ˮ�������Ƿ�ֹ�����ۻ����¶ȹ��߶�ը�Ѽ���ƿ��̽��ȼ�յ���������ˮ�Ǽ���ͭƬ�¶ȣ�����������
��� �⣺
��1����������ָ��һ��������ɵ����ڴ��������ˮ�к�������������ˮ�к����Ȼ��Ƶ����ʣ�
��ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ��Ҫ���������غ㶨�ɣ���Ӧǰ��Ԫ������䣬������������ȼ������ˮ��ˮͨ����������������������˵��ˮ�к�����Ԫ�غ���Ԫ�أ�
��2��A��ˮ���Ӳ����˶����ù۵���Ϸ����Dz����˶����ص㣬��A��ȷ��
B��ˮ����֮�������䣬ˮ������������ˮ���ӱ���û�иı䣬�ı���Ƿ��Ӽ�ļ��������ˣ���B����
C���⡢��ԭ�Ӳ������ı䣬ˮ�����������仯��ˮ���Ӳ��䣬���е���ԭ�Ӻ���ԭ��Ҳ���䣬��C��ȷ��
D�������DZ������ʻ�ѧ���ʵ���С���ӣ������ʱ��ָ�С������ʱ��ֻ�ܱ������ʵĻ�ѧ���ʣ��������ٱ������ʵ��������ʣ���D����
ͨ����ˮ�������ɵ��������������������ɵ����������������������Ϊ��1��2��ˮ���ķ���ʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�����Թ�b��������������
��3��С��ʯ��ʯӢɳ������������ȥ������ˮ�����ʣ���������С��ʯ��ʯӢɳ�������������ǹ��ˣ�����̿�����������ã���������ˮ�е�ɫ�غ���ζ�����÷���ˮ�ж����õ�ˮ��Ӳˮ������ˮ�����в�����ĭ�϶������ˮ��������ĭ���ٵ���Ӳˮ��
��4������������ȼ�����ɶ���������������Ⱦ���������ˮ���������ն�������ģ��ⶨ����������������ˮ������ͨ��ˮ����ı仯��չʾ�����ĵ��������������˿��������ȼ�գ�����ƿ�м���ˮ�������Ƿ�ֹ�����ۻ����¶ȹ��߶�ը�Ѽ���ƿ��
�𰸣�
��1������ˮ��AC��
��2��AC��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����O2��
��3�����ˣ��������������ˮ��������ĭ�϶������ˮ��������ĭ���ٵ���Ӳˮ��
��4�����ն��������ֹ������Ⱦ��չʾ�����ĵ��������������ֹ���������ヲ��ը��ƿ�ף�
���� ��֪ʶ�Ĺ��ɡ���������Ч��ѧϰ������ͨ�������֪ʶ�Ĺ��ɡ�����������Ч�ذ���ɢ֪ʶϵͳ���������ڶ���������Ľ��


 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �÷�Ӧ�����������ڿ�����ȼ�� | |
| B�� | �ڻ�ѧ��Ӧǰ��ԭ�ӵ�����û�иı� | |
| C�� | ��ѧ��Ӧ�з��ӿɷ�Ϊԭ�� | |
| D�� | �û�ѧ��Ӧ���ڷֽⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  Ϩ��ƾ��� | B�� |  �������� | ||
| C�� |  ���� | D�� | 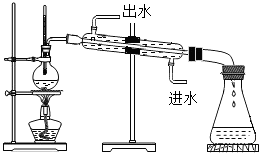 ��ȡ����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ֱ�ͨ������ʯ��ˮ�� | B�� | �۲��������ɫ | ||
| C�� | ��ȼ�յ�ľ���ֱ����뼯��ƿ�� | D�� | ������ֱ�ͨ��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� | |
| �� | 50.0g | 1% | 0.1g | 9mL |
| �� | 50.0g | 2% | 0.1g | 16mL |
| �� | 50.0g | 4% | 0.1g | 31mL |
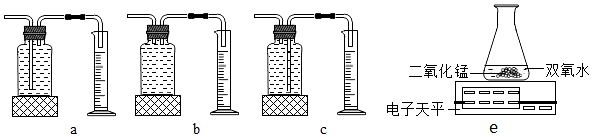
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼ | B�� | ���� | C�� | ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
 �����еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺
�����еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ��塲Ca10��PO4��6��OH��2����ʽ���ڣ�ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com