
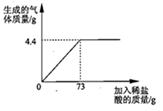
 ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ
ijƷ�ƴ����к�������NaCl����ѧ��ȤС���ͬѧ����������ʵ��̽������ȡ12g��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ�������Ƴ�����ϡ�����������ų����������Ĺ�ϵ��ͼ
��1������ǡ����ȫ��Ӧʱ������CO2������Ϊ g
��2���������Ʒ�к����ʵ��������� �Ƕ��٣�������������һλС������ͬ��
�Ƕ��٣�������������һλС������ͬ��
��3�����㵱�����봿 ��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
��ǡ����ȫ��Ӧʱ��������Һ���������������Ƕ��٣�
���𰸡���1��4.4
��2���⣺����Ʒ��̼���Ƶ�����Ϊx����ȫ��Ӧ�����Ȼ��Ƶ�����Ϊy
Na2CO3 + 2HCl === 2NaCl + H2O + CO2��
106 117 44
x y 4.4g
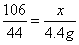
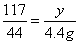
��ã�x=10.6g y=11.7g
������Ʒ���Ȼ��Ƶ�����Ϊ12g-1.06g=1.4g
�Ȼ��Ƶ���������Ϊ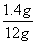 ��100��=11.7��
��100��=11.7��
��3����Ӧ����Һ������Ϊ73g+12g-4.4g=80.6g
1.4g+11.7g=13.1g
����������Һ��������������Ϊ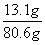 ��100��=16.3��
��100��=16.3��
����Ʒ�������Ȼ��Ƶ���������Ϊ11.7��������ǡ����ȫ��Ӧʱ��������Һ��������������Ϊ16.3����
����������ͼ��ֱ�ӻ�ȡ��ȫ��Ӧ���ɶ�����̼������Ϊ4.4g�����ö�����̼�������ɼ����̼���Ƶ��������ٸ�����Ʒ��������������Ȼ��Ƶ���������������غ㶨�ɿɼ������Ӧ��������Һ���������������ɶ�����̼�������ɼ������Ӧ�����Ȼ��Ƶ����������������Һ����������������


 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼��̼�Ļ���������������������Ӧ�ù㷺�����ſƼ��IJ���ӿ�֡��������ѧ֪ʶ�ش��������⣺
(1)���к�̼Ԫ�ص������У������л������____(����ĸ���)��
A��̼��� B���Ҵ� (C2H5OH) C��һ����̼

(2)��ͼ��̼���ࡱ��Ŀǰ��֪����Ĺ�����ϣ� ֻ��̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������(����ˮ)���������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵������ȷ����____(����ĸ���)��
A������������
B�����ظ�ʹ��
C���ɴ�������й©��ʯ��
D�����ڸ��ϲ���
(3)��������̼�������Ҫ��Ϊ�˼���__ __(�ѧʽ)���ŷ��������һ�����ճ������з�����һ���������__ __��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ�������˳����ͭ����������������ģ���������õĴ������ޡ�

��1��������ͼ�Ĺ��ɣ�����Ϊ���������ģ���������õ��Ⱥ�˳����Ҫ�� �йء�
��2����ѧС���ͬѧ��ģ��ʪ����ͭʵ���еõ�������ͭ����������к����ⶨ����ȡ10�˹������������ձ��У��μ�һ������������ϡ������Һ����¼����������ͼ��ʾ�����ߡ�
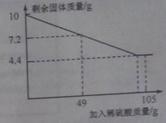
��10�˹���������ͭ������Ϊ �ˣ�
������ϡ������Һ���ʵ����������Ƕ��٣���д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
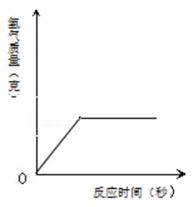
100��ijŨ�ȵ�����ǡ����13�˵�п��ȫ��Ӧ������㣺
����������������(�����ȷ��0.1g)��
�ƽ��ŵĽ������ͼ�У�
�Ƿ�Ӧ��������Һ�����ʵ���������(д��������̣������ȷ��0.1%).
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij������к���̼��ƺ��Ȼ��ƣ�ȡ�û����6g�������м���һ����������������Ϊ10%��ϡ���ᣬǡ����ȫ��Ӧ������2.2g���塣����������ȫ���ݳ�����
���㣺
��1�� ��ȡ�������̼��Ƶ�������
��2�� ����ϡ���������
��3�� ��Ӧ��������Һ�е�������������������ȷ��0.1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��һ������Ũ����Ĺ�����������������������Ϊ98%��Ũ����20tй©������·���������ӡ��ӵ������������ٱ�������������ʯ�ҽ�����Ҫ�ɷ�Ϊ�������ƣ��к������������顣��ش�
��1��������������Ϊ98%��Ũ����20t�к�H2SO4�������� ��
��2�������к�й©��98%Ũ����20t����������Ҫ�������Ƶ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20g�������Ƭ����50gϡ�����У�ǡ����ȫ��Ӧ���ų����������Ϊ0.4g������
��1����Ƭ�е�����������
��2����Ƭ���������������
��3�����������Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧ������ũ�壬����ʹ�õ��Ǿ�ˮ���տ�ˮ�Ĵ�����һ��ܺ��ˮ�����������Ϻ��֪��ˮ������Ҫ�ɷ���CaCO3��Mg��OH��2����Ϊ��Ū��ˮ����̼��Ƶ�����������ȡ��������ˮ����Ʒ����ʵ������ϡ�������䷴Ӧ�����з�����
��1��ʵ���ҵ�ϡ����������������Ϊ38%��Ũ�������Ƶģ�����1000g��������Ϊ10%��ϡ������Ҫ��Ũ���������Ϊ�� ��g������һλС������
��2��Сλȡ10gˮ����Ʒ��������ϡ�����ַ�Ӧ�õ�3.3g������̼����ˮ����̼��Ƶ���������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡ����ʱ���õ�װ��(����G��IΪ����װ��)������װ�ûش��������⣺
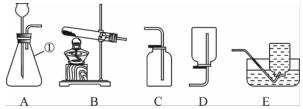
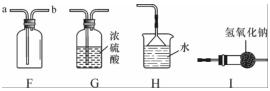
(1)�����ٵ����ƣ�______��
(2)��Aװ����ȡ��ij�����ʹ�����ǵ�ľ����ȼ����д����Aװ����ȡ������Ļ�ѧ����ʽΪ__________________________________ ________��
________��
(3)����Cװ���ռ�һƿ��ɫ��ζ�����壬���������Ƿ�ΪCO2�ķ�����__________________��������Fװ���ռ������壬������Ӧ��______(�a����b��)ͨ�롣
(4)�����£�����(NH3)��һ���д̼�����ζ�����壬��������ˮ���ܶȱȿ���С����Һ�ʼ��ԡ�ʵ������ȡ������ԭ����NH4Cl(��)��Ca(OH)2(��)��Ϲ��ȡ���Ҫ�Ƶø���İ���������β������ֹ��Ⱦ��������ѡ���װ������˳��������B��(______)��F��H(��װ�����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com