
A��B��C��D��E�Ǿ��꼶��ѧ�ϲ᳣�����������ʣ���������ͼ��ʾ��ϵ����֪A��B��EΪ�����C��DΪ���ʣ���A��B��Ӧ��B��C��Ӧ������һ�ֺ�ɫ���ʡ��������ͼʾ�����������ش���������:
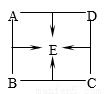
��1��A���ʵĻ�ѧʽΪ__________��D���ʵĻ�ѧʽΪ_____________��
��2��B��C��Ӧ�Ļ�ѧ����ʽ��______________________________��
��3��E��C�ڸ���������Ҳ���Է�����ѧ��Ӧ��ֻ����һ���ж������ʣ� �÷�Ӧ�Ļ�ѧ����ʽ��_________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ���꼶3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A����ˮ�ľ����У����������̿��������ȫ��ͬ
B�����ͺ�ϴ�ྫ���ɳ����ۣ������ԭ����ȫ��ͬ
C����KClO3��˫��ˮ��O2ʱ�������MnO2������ͬ
D����¯����������ĸ��º�CO�����ɶ��뽹̿�й�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�����о��꼶�п���ϰ������ϰ9��������ʡ���ѧ�Ծ��������棩 ���ͣ�ѡ����
Ũ�����Ũ���᳨�ڷ����ڿ�����һ��ʱ�����Һ�У� ��
A�����ʵ�������������С
B����Һ������������
C�����ʵ��������������
D�����ʵ���������ǰ�߱���߱�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡǭ������������������꼶��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ�������
�ڷ�Ӧ3Cu��8HNO3��3Cu(NO3)2��2X����4H2O�е�X�Ļ�ѧʽ�ǣ� ��
A. N2 B. NO C. NO2 D. N2O5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡǭ������������������꼶��ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ�ѡ�������
����ʵ������У����߱���Ӵ����� �� ��
A. ���Թ��м���Һ���Լ�ʱ���Լ�ƿ�����Թܿ�
B. ���Թ��еμ��Լ�ʱ���ι����Թ�
C. �þƾ��Ƹ��Թܼ���ʱ���Թ���ƾ���
D. �þƾ��Ƹ��Թܼ���ʱ���Թ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����о��꼶��ѧ����ĩ��ѧ������⻯ѧ�Ծ��������棩 ���ͣ���Ϣ������
�Ӣ������ڵ�����������һ����̼�ݶ�����������������У�ѡ������������������ʣ��������ա�
��1��δ�������������ȼ����_____����2��ҽ������������Σ�ز��˵���_____��
��3����Ⱦ�������γ��������_____����4�����̲�����һ���к�����______��
��5����ʳƷ��װ���У�����ʳƷ����������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����о��꼶��ѧ����ĩ��ѧ������⻯ѧ�Ծ��������棩 ���ͣ�ѡ�������
����������������Ԫ�ؾ��з������������á��������ͼ������Ϣ�ж�����˵����ȷ����
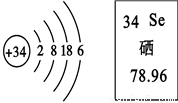
A. ��Ԫ�����ڽ���Ԫ��
B. ��ԭ�ӵ����ԭ������Ϊ78.96 g
C. �ڻ�ѧ��Ӧ�У���ԭ����ʧ����
D. ��ԭ�ӵ�������Ϊ34
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ����������Ҫ��Ƭ���꼶��һ��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��ѧ�벻��ʵ�飬������ʵ���Ҳ���������װ����ͼ��ʾ����ش��������⣺
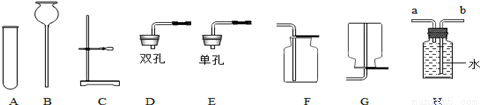
��1��B������������_________________��
��2����Ҫ��װһ������̼�ķ���װ�ã���ѡ��ͼ�е�_____________���������·�����ĸ����ͬ�����ռ�װ�ÿ�ѡ��ͼ�е�________��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ_______________________���ø÷���װ�û�������ȡ��������________����һ�֣���
��3�����ø��������ȡ�������������Ӿƾ����⣬����Ҫ��ʵ����Ʒ��______��д���ø��������ȡ�����Ļ�ѧ����ʽ___________________����ͼH��ʾװ���ռ�����������Ӧ��______���a����b�����˵��롣
��4������������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����
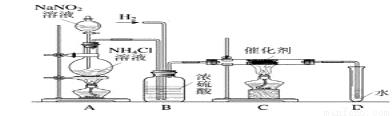
��Cװ�õ�Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ___________________________��
�ڷ�ӦʱN2��H2�������������__________��������˱������з�Ӧ����Ӧʱ��D�е��ܿ��Ƿ���ݳ����ݣ�����֪NH3��������ˮ����˵���ݳ����ݵ�ԭ��_____________��
����ʵ�鷽��֤��ȷʵ��NH3���ɣ� _______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ�Ͼ�����������꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����±��ش����⣺
�¶ȣ��棩 | 20 | 40 | 50 | 60 | 80 | |
�ܽ�� ��g�� | NaCl | 36.0 | 36.6 | 37.0 | 37.3 | 38.4 |
NH4Cl | 37.2 | 45.8 | 50.4 | 55.2 | 65.6 | |
KNO3 | 31.6 | 63.9 | 85.5 | 110 | 169 | |
��1��20��ʱ���ܽ������������______________��
��2��40��ʱ��100gˮ������ܽ�NaCl______________g��
��3������80�溬��110gˮ��KNO3��Һ���������²������õ�20gKNO3���塣
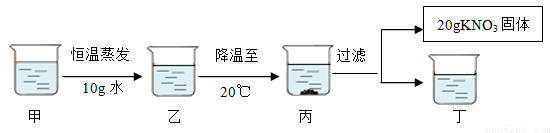
�ٶ���ҺΪ_______________��ѡ����͡������͡�����Һ��
�ڶ����Ϲ��̵ķ�������ȷ����_____________������ĸ����
A�����ҵĹ����У���������û�иı�
B�������������ܼ���������Ϊ169��100
C����ʼ����KNO3������¶���40����50��֮��
D������Һ����������161.6g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com