
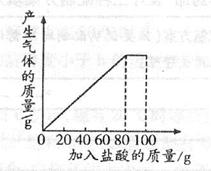
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35g��ʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��
| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��1��25��2��57��1% ��3��163��5g
���������������1��û�м�����ǰ�����������Ϊ35g����һ�μ���������������������5g������Ӧ��5g̼��ƣ���ô��2�μ���������ֻᷴӦ5g̼��ƣ������������Ϊ25g����aΪ25��
��2������ͼ���������4�μ����������Ʒ��̼�����ȫ��Ӧ����ʱʣ��Ĺ���SiO2����Ϊ15g����̼��Ƶ�����Ϊ35g-15g=20g,��ʯ��ʯ��Ʒ��̼��Ƶ���������=20g/35g��100%=57��1%
��3����ͼ����֪��ÿ����20g���ᣬ������5g CaCO3����100g��������25g CaCO3��
��100g������ȫ��Ӧ����Һ��CaCl2������Ϊx������CO2������Ϊy
CaCO3 + 2HCl === CaCl2 + H2O + CO2��
100 111 44
25g x y
100/111 = 2g5/x x = 27��75g
100/44 = 25g/y y=11g
����CaCl2��Һ������Ϊ25g+100g-11g=114g
�軹��Ҫ��ˮ������Ϊz
27��75g/(114g+z) ��100%=10%
z=163��5g
�𣺣�1��aΪ25g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ57��1%��
��3������Ҫ����Һ�м���163��5gˮ��
���㣺���û�ѧ����ʽ�ļ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ȤС����10gþ����������������Ϊ49%��ϡ���ᷴӦ����ò���������������ϡ�����������ϵ��ͼ������þ���г����溬������þ�⣬û���������ʣ�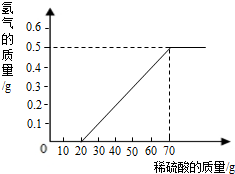
��1����ϡ���������Ϊ70g������������������Ϊ0.5g
��2���û�ѧ����ʽ˵����ϡ���������Ϊ10gʱ��Ϊʲô���������� ��
��3������þ����þԪ�ص�����������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�28.7g���Ȼ��ƺ������ƵĻ�������100gˮ�У���������Һ�м���134g����������Һ��ǡ����ȫ��Ӧ���õ���Һ��������234g�����㣺ԭ���������Ԫ�ص���������������֪��AgNO3 + NaCl = AgCl��+ NaNO3����������ȷ��1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�ȡ24 g�������ƺ�̼���ƵĹ��������136 gˮ��ȫ�ܽ���ٻ�������ʯ���飨�������ƺ�ˮ�Ļ�����ǡ����ȫ��Ӧ�����˵õ�4 g������10%������������Һ���Լ��㷴Ӧ��Ӧ����ʯ����������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��117.0g�Ȼ�����Һ��140.0g����������Һ��ϣ�ǡ����ȫ��Ӧ�����˺�������Һ������Ϊ228.3g��������Ȼ�����Һ����������������
����Ӧ�Ļ�ѧ����ʽΪNaCl+AgNO3=AgCl��+NaNO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ�ⶨij����(Na2CO3)��Ʒ�У������������Ȼ������ʣ�̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ��ϡ����μ���36��5gʱ���ձ�����Һ��������Ϊ40��3g������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺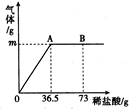
��A��������������Ϊ ��
��������̼���Ƶ����������������ȷ��0��1%����
��B��ʱ���ձ�����Һ�����ʵĻ�ѧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ���Ҳⶨһƿϡ���������ʵ�����������ȡ10g�ô���Һ����������μ���5����NaOH��Һ������NaOH��Һ����������ҺpH�ı仯��ϵ��ͼ��ʾ��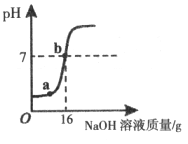
��1��16g 5����NaOH��Һ���������ʵ�������________g��
��2��a���Ӧ��Һ�е�������________________��
��3���������ϡ���������ʵ�����������д��������̼��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ѧʵ���У����õ��ˮ�ķ����Ʊ����������������о�����ʹ�ã��ֵ��1.8�Kˮ�����Ƶ������������� �K
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��������2%��ϡ����ⶨ�����ŷŵķ�ˮ���������صĺ���(��ˮ�е��������ʲ���ϡ���ᷴӦ)���Լ��㣺��ʢ��20 g��ˮ����ƿ����εμ�2%��ϡ���ᣬ��ǡ����ȫ��Ӧ����ʵ���������ݻ��Ƴ���ͼ��ʾͼ�����ˮ���������ص�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com