
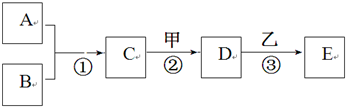
A~E���ס��Ҿ��������л�ѧ�еij������ʡ�������һ�������µ�ת����ϵ��
ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е�
�������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�dz����ڸ������������ļ
 ��ش�
��ش�
��B���ʵĻ�ѧʽ���������� ������D���ʵ��׳������������������� ����дһ�֣�
��д����Ӧ�ڵĻ�ѧ����ʽ������������������������������������������������ ��
��������E���д̼�����ζ�����壬�������ʿ��������������������� ����дһ�֣�
������E����ɫ��������D��Һ������Һ�����ķ�Ӧ�۵Ļ�ѧ����ʽ�����ǣ�
�������������������������� ���������������������������������� ����дһ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ش�
��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ̩����ѧ�������о��꼶��ѧ�ڵ�Ԫ��⻯ѧ�Ծ� ���ͣ������
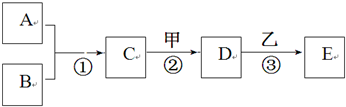
A~E���ס��Ҿ��������л�ѧ�еij������ʡ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ������������ЧӦ����Ҫ���塣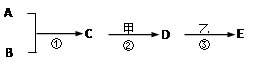 ��ش�
��ش�
��1��B���ʵĻ�ѧʽ���� ����D���ʵ��׳� ����дһ�֣�
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��CҲ��ת��ΪA��B��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�����ʡ�人�г����һѧУ�п���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com