��2013?������һģ����������CO
2��������̼�����չ��̼�����ѳ�Ϊһ�ֻ���ʱ�У�
��1������̼���С��Ľ�������˶�����̼������ŷţ��ܼ���
����ЧӦ
����ЧӦ
�ij̶ȣ�
��2��2014����»ἴ�����Ͼ����У�Ϊ�˿�������β����������ɵ���Ⱦ���Ͼ�����ȼ�ϵ�ʹ�ú����ȷ����ȡ��һЩ��Ч��ʩ��ͼ1�й�����ʹ�õ�ȼ����Ҫ�ɷ�����Ȼ��������ȫȼ�յĻ�ѧ����ʽΪ
��
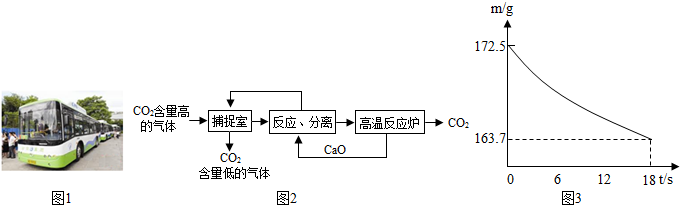
��3�������п�ѧ��������á�̼��������������ҵ�����ж�����̼���ŷ�������̼����������ָͨ��һ���ķ���������ҵ�����в�����CO
2����������д�������ã�������������NaOH��Һ��������CO
2��������ͼ2��ʾ����������������δ�������
�ٲ����з�����Ӧ�Ļ�ѧ����ʽΪ
CO2+2NaOH�TNa2CO3+H2O
CO2+2NaOH�TNa2CO3+H2O
��
�ڰ�CaO���뷴Ӧ����������H
2O��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
�����ô˷�Ӧ�������ƿ�����ʳƷ
����
����
����
�ۡ���Ӧ���롱�У��õ��������ʵĻ���������
����
����
���ù�����̼��ƣ�
�����������У�����ѭ�����õ�������
CaO��NaOH
CaO��NaOH
��
��4����ȡij������Ʒ21.5g�����뵽ʢ������ϡ������ձ��У��������ò��������裮��Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ��ͼ3��ʾ��
| ��ҵ���������Na2CO3%�ݣ� |
| �ŵ�Ʒ |
һ��Ʒ |
�ϸ�Ʒ |
| 99.2% |
98.8% |
98.0% |
��ͨ�����㲢���ͼ���ش�
�ٷ�Ӧ����CO
2������Ϊ
8.8
8.8
g��
���жϴ˴�����Ʒ�ĵȼ���
������̣�




