



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
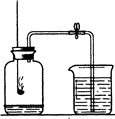 £Ø2009?ŌĄŃō£©ÓĆČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆĄ“²ā¶ØæÕĘųĄļŃõĘųµÄŗ¬Į森Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø2009?ŌĄŃō£©ÓĆČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆĄ“²ā¶ØæÕĘųĄļŃõĘųµÄŗ¬Į森Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 “󶬻įĘŚ¼ä£¬¹ž¶ū±õŹŠ¾ŁŠŠĮĖĀĢÉ«»·±£ŠĶ¹«½»³µ·¢³µŅĒŹ½£®ŅŌŃ¹ĖõĢģČ»ĘųĪŖ¶ÆĮ¦”¢·ūŗĻ¹ś¼ŹÅŷűź×¼µÄ¹«½»³µ¼“ČÕĘšĶ¶ČėŹ¹ÓĆ£¬³ÉĪŖµŚ24½ģ“󶬻įĘŚ¼äĮ¬½Ó¹ž¶ū±õŹŠÄŚø÷±ČČü³”¹ŻµÄÖŲŅŖ½»Ķع¤¾ß£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
“󶬻įĘŚ¼ä£¬¹ž¶ū±õŹŠ¾ŁŠŠĮĖĀĢÉ«»·±£ŠĶ¹«½»³µ·¢³µŅĒŹ½£®ŅŌŃ¹ĖõĢģČ»ĘųĪŖ¶ÆĮ¦”¢·ūŗĻ¹ś¼ŹÅŷűź×¼µÄ¹«½»³µ¼“ČÕĘšĶ¶ČėŹ¹ÓĆ£¬³ÉĪŖµŚ24½ģ“󶬻įĘŚ¼äĮ¬½Ó¹ž¶ū±õŹŠÄŚø÷±ČČü³”¹ŻµÄÖŲŅŖ½»Ķع¤¾ß£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ēė½āŹĶĻĀĮŠĒéæö£ŗ

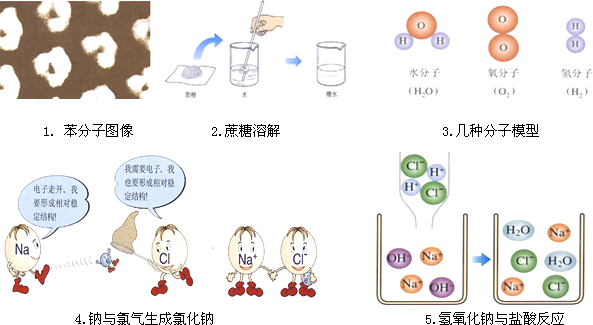
1. ±½·Ö×ÓĶ¼Ļń 2.ÕįĢĒČܽā 3.¼øÖÖ·Ö×ÓÄ£ŠĶ
(1)Ķ¼Ņ»ÖŠ“ÓĪ¢¹Ū½Ē¶ČÄć»ńµĆµÄŅ»ĢõŠÅĻ¢ ”ų ”£
(2)Ķ¼¶žĖłŹ¾ĻÖĻóµÄ½āŹĶ ”ų ”£
(3)¾ŻĶ¼ČżĖłŹ¾£ŗČē¹ū½«Ė®ĶصēøĆ·“Ó¦µÄĪ¢¹Ū¹ż³ĢŹĒ£ŗ¢ŁĖ®·Ö×Ó·Ö½āĪŖĒāŌ×ÓŗĶŃõŌ×Ó£»¢Ś±ķŹ¾ĪŖ ”ų £»¢Ū“óĮæµÄŃõ·Ö×Ó¾Ū¼Æ³ÉŃõĘų£¬“óĮæµÄĒā·Ö×Ó¾Ū¼Æ³ÉĒāĘų”£


4.ÄĘÓėĀČĘųÉś³ÉĀČ»ÆÄĘ 5.ĒāŃõ»ÆÄĘÓėŃĪĖį·“Ó¦
(4)Ķ¼4æØĶØÖŠĖłĖµµÄĪČ¶Ø½į¹¹£ŗÖøµÄŹĒ×īĶā²ćÓŠ ”ų µē×ӵĽį¹¹”£
(5)øł¾ŻĶ¼5²Ā²āĒāŃõ»ÆÄĘÓėĮņĖį·“Ó¦ŗóČÜŅŗÖŠµÄĮ£×ÓÓŠ ”ų ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com