

 =
=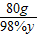
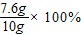 =76%£®
=76%£®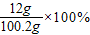 ”Ö12.0%£®
”Ö12.0%£®

 æĪĢĆČ«½ā×Ö“Ź¾ä¶ĪĘŖÕĀĻµĮŠ“š°ø
æĪĢĆČ«½ā×Ö“Ź¾ä¶ĪĘŖÕĀĻµĮŠ“š°ø ²½²½øßæŚĖćĢāæØĻµĮŠ“š°ø
²½²½øßæŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗÄĻøŚĒų¶žÄ£ ĢāŠĶ£ŗµ„Ń”Ģā
| A£®±»ĪĆ³ę¶£Ņ§ŗó£¬æÉĶæ·ŹŌķĖ®¼õĒįĶ“Ń÷ |
| B£®ÓƶžŃõ»ÆĢ¼Ćš»šĘ÷ĘĖĆšĶ¼Źéµµ°ø”¢¹óÖŲÉč±øµČµÄŹ§»š |
| C£®ÓĆFVCÖĘ³ÉŹ³Ę·°ü×°“ü£¬ĪŖČĖĆĒµÄÉś»īĢį¹©ĮĖ·½±ć |
| D£®Ņ½ĮĘÉĻæÉŌŚŅŗµŖĄä¶³Āé×ķĢõ¼žĻĀ×öŹÖŹõ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÖŠæ¼»ÆŃ§Ä£Äā³å“ĢŹŌ¾ķ£ØŹ®Ņ»£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠĻć·»ĒųÖŠæ¼»Æѧ¶žÄ£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØĪ壩£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2008ÄźŗŚĮś½Ź”¹ž¶ū±õŹŠÖŠæ¼»ÆŃ§Ä£ÄāŹŌ¾ķ£ØŅ»£©£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com