
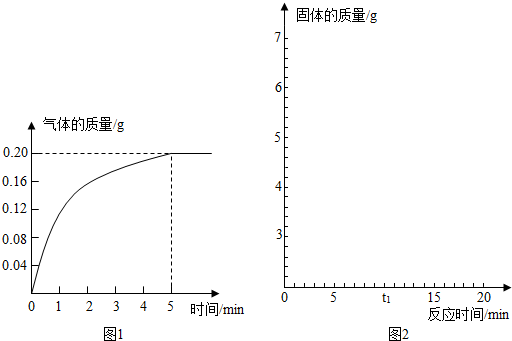
| 56 |
| x |
| 2 |
| 0.2g |
| 10g-5.6g |
| 10g |
| 56 |
| 2.8g |
| 64 |
| y |




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ��������ȼ����� |
B�� ���վ�����ϩ���ϲ���ȫȼ�� |
C��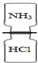 ��ϰ������Ȼ������� |
D�� �ڿ����е�ȼþ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ֲ��ĺ��� | B���������� |
| C����ɽ��DZˮ | D����ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����-d | B����-a |
| C����-a | D����-b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʪ���·���� |
| B��ʯ���ۻ� |
| C��ͭ�ڳ�ʪ�Ŀ���������ͭ�� |
| D�����ͻӷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2NO����2����ʾ2��һ���������� |
| B��Mg2+����2����ʾþԪ�صĻ��ϼ�Ϊ+2 |
| C��2O����2����ʾ2�������� |
| D��H2O����2����ʾ1��ˮ����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ֻ�����֮�䷢���ķ�Ӧ�����кͷ�Ӧ |
| B�����ֽⷴӦһ�����кͷ�Ӧ |
| C�������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ |
| D���������кͷ�Ӧ������һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| CaCl2��Һ������/g | 25 | 50 | 75 | 100 | 125 | 150 |
| ����������/g | 2 | 4 | m | 8 | 10 | 10 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com