

���� ��1�����ݷ�Ӧ���״̬�뷴Ӧ����ȷ����ȡװ�ã����ݻ�ѧ��Ӧԭ���Լ���ѧ����ʽ��д����������
��2�������ռ�װ�÷�����������ʣ����������Ƿ�ͨ����ѧ�仯�����ַ������������ʻ��ǻ�ѧ���ʣ�
��3�����������������¶ȵĹ�ϵ�����������������ݷ�Ӧ�������ͷ�Ӧ����д����Ӧ�ķ���ʽ��
��� �⣺��1��������غͶ������̵Ļ������ȡO2�����ڹ�������ͣ�����ѡ��װ��A����ȡ��������ڶ������̵Ĵ������¼��ȷֽ�Ϊ�Ȼ��غ����������A��2KClO3$\frac{\underline{\;MnO_{2}\;}}{��}$2KCl+3O2����
��2�����Թܢ��ռ�CO2������ΪCO2���ܶȱȿ������ܶȴ����ʲ���Ҫ������ѧ�仯�����������������ʣ�����ܶȱȿ�����
��3���������������¶ȵ����߶�����������ʹCO2�������װ�г���ʯ��ˮ���ձ��У�Ӧ���еIJ����Ǵ�K2���ȣ���˫����ס���Թܢ٣�������̼��ʹ�����ʯ��ˮ����ǣ���Ӧ�ķ���ʽ��CO2+Ca��OH��2�TCaCO3��+H2O�������K2���ȣ���˫����ס���Թܢ٣�CO2+Ca��OH��2�TCaCO3��+H2O��
���� ������Ҫ�����˳��������װ�õ�ѡȡ������Ҫ�����������Ŀ����Ҫ��dz�������ķ���װ�ú��ռ�װ����ѡȡ�����������ݣ������������ȡԭ������װ��ѡ�䡢�жϻ���ѡ������ķ���װ�ã��ٸ���������ܽ��ԡ��ܶȼ����Ƿ���ˮ���߿���������Ӧ�����жϡ�ѡ���ռ�װ�ã��������ۡ�����ijһװ�õȵȣ�


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢۢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
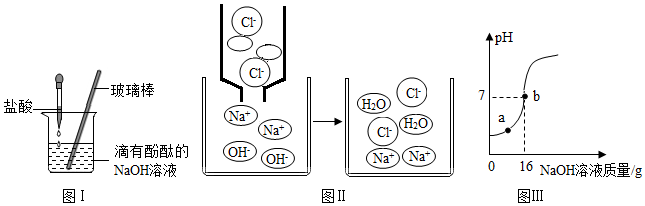
 ������д�����ӷ���ΪH+��
������д�����ӷ���ΪH+���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com