
A~F�dz��г��������ʡ�
��1��A������������ȼ�ϣ���A�Ļ�ѧʽ��______��
��2��B��һ�������·ֽ��A����һ�����壬�÷�Ӧ�Ļ�ѧ����ʽ��______��
��3��D��������Ԫ����ɵ������Թ��壬��E��Һ��Ӧ��������F ����D��F���
����D��F���
ת������Fת��ΪD�Ļ�ѧ����ʽ��______��
��4������ʱ��3 g������һ������B��Ӧ���õ�ֻ������Ԫ�صĺ�ɫ�����0.1 g A�����ɫ�����и�Ԫ�ص���������______���������������ȱ�ʾ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������װ����ȷ����
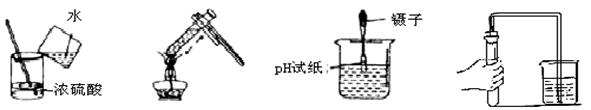
A��ϡ��Ũ����  B������Һ�� C���ⶨ��Һ��pH D�����װ�õ�������
B������Һ�� C���ⶨ��Һ��pH D�����װ�õ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ɭ��ͻ�����ʱ��������Ա��ʹ��һ���ֳ�ʽ����������������������ٿ����������𡣸����������ԭ����
| A���������� B�����߿�ȼ�� C�����Ϳ�ȼ����Ż�� D�����µ��Ż������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�þƾ��Ƽ���һ�������ĸ�����ء�����ͼ���ʾ�ù�����ijЩ����ʱ��ı仯���ƣ�������ȷ����
 |
A B C D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ���̼��ԭ����ͭ��ʵ��õ�����ɫ��Cu�Ͱ���ɫ��Cu2O�Ļ����ֽ�8.4 g�û��������ձ��У��������ϡ�����ַ�Ӧ���ˡ�ϴ�ӡ�����õ�6.4 g���塣����ԭ�������Cu2O��������
 ��֪��Cu2
��֪��Cu2 O + H2SO4 CuSO4 + Cu + H2O
O + H2SO4 CuSO4 + Cu + H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ����15%���Ȼ�����Һ50 g��
(1)����:���Ȼ��Ƶ�����Ϊ����������
(2)ijͬѧ����ͼ��ʾ�������β���:

���в�������IJ�����������(����ĸ���);B������������Ϊ��������;E�в��������������� ��
(3) ����ͼA�����������Ϊ5 g,����Ķ���Ϊ2.5 g,��С���Ƶõ��Ȼ�������ʵ��Ϊ������ ����
����ͼA�����������Ϊ5 g,����Ķ���Ϊ2.5 g,��С���Ƶõ��Ȼ�������ʵ��Ϊ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C��O��Ca�dz��л�ѧ����������Ԫ��,��ѡ�����е�Ԫ��д����������Ҫ������ʵĻ�ѧ ʽ:
ʽ:
(1)ʯī__________________________________________________________��
(2)��֧��ȼ�յ� ����______________________________________________��
����______________________________________________��
(3)��ȼ�յ�����__________________________________________________��
(4)��������������______________________________________________��
(5)��������ȡ�� ����̼����İ�ɫ�������Ҫ�ɷ�____________________
����̼����İ�ɫ�������Ҫ�ɷ�____________________
______________________________________________��
(6)����������____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
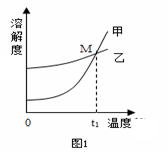 K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�����ݼ���Ӧ���ܽ����������
K2CO3��KNO3�ڲ�ͬ�¶�ʱ���ܽ�����ݼ���Ӧ���ܽ���������� ��
��
| �¶�/�� | 20 | 30 | 50 | 60 | 80 | |
| �ܽ�� (g/100gˮ) | K2CO3 | 110 | 114 | 121 | 126 | 139 |
| KNO3 | 31.6 | 45.8 | 85.5 | 110 | 169 |
��ͼ1�б�ʾKNO3�ܽ�����ߵ�����24������ס����ҡ�����
��������M������ ��25�� ��
��ͼ1��t1���¶ȷ�ΧΪ ��26�� ��
��20��ʱ��60g K2CO3������뵽50��ˮ�У��õ�����Һ������������������27�� ��
��80�� ʱ��KNO3������Һ��������K2CO3������Ҫ�õ��ϴ�����KNO3���壬�˲���ʵ����������� ��28�� ��
��40�� ʱ���������ֱ�ʢ����ͬ�������� ��غ�̼��ص��ձ��У�������100gˮ������ܽ�ָ���40�� ��������ͼ2��ʾ���й�˵���У���ȷ���� ��29�� ��������ĸ��
��غ�̼��ص��ձ��У�������100gˮ������ܽ�ָ���40�� ��������ͼ2��ʾ���й�˵���У���ȷ���� ��29�� ��������ĸ��
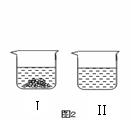 A���ձ�II����Һ�Dz�������Һ
A���ձ�II����Һ�Dz�������Һ
B���ձ�I���ܽ����KNO3���ձ�II���ܽ����K2CO3
C�������¶Ȼ������ܼ����п��ܽ��ձ�I�й���ȫ���ܽ�
D�������ձ�I�е���Һ��Ϊ��������Һ����Һ��������������
һ����С
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com