
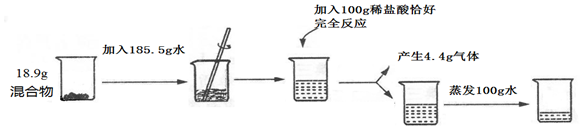
·ÖĪö £Ø1£©øĆŹµŃéĢ¼ĖįÄĘÓėŃĪĖį·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĖ®ŗĶĀČ»ÆÄĘ£¬øł¾ŻÖŹĮæŹŲŗć¶ØĀÉÕżČ·ŹéŠ“»Æѧ·½³ĢŹ½£»
£Ø2£©øł¾Ż»Æѧ·½³ĢŹ½ÖŠø÷ĪļÖŹµÄÖŹĮæ±Č·ÖĪö½ā“š£»
£Ø3£©øł¾Ż»Æѧ·½³ĢŹ½£¬½įŗĻ¶žŃõ»ÆĢ¼µÄÖŹĮæĄ“¼ĘĖć³öĢ¼ĖįÄʵÄÖŹĮ漓æɽā“š£»
£Ø4£©ĒóĖćĖłµĆČÜŅŗÖŠĀČ»ÆÄʵÄ×ÜÖŹĮæŗĶĖłµĆČÜŅŗµÄÖŹĮ棬½ų¶ųĒóĖćÖŹĮæ·ÖŹż£»
£Ø5£©ČÜŅŗĻ”ŹĶ¹ż³ĢÖŠČÜÖŹµÄÖŹĮæ±£³Ö²»±ä£¬¾Ż“Ė¼ĘĖć£»
½ā“š ½ā£ŗ£Ø1£©øĆŹµŃéĢ¼ĖįÄĘÓėŃĪĖį·“Ӧɜ³É¶žŃõ»ÆĢ¼ŗĶĖ®ŗĶĀČ»ÆÄĘ£¬»Æѧ·½³ĢŹ½ĪŖ£ŗNa2CO3+2HClØT2NaCl+H2O+CO2”ü£»
£Ø2£©ÉčĢ¼ĖįÄʵÄÖŹĮæĪŖx£¬ĻūŗÄĀČ»ÆĒāµÄÖŹĮæĪŖy£¬Éś³ÉĀČ»ÆÄʵÄÖŹĮæĪŖz£¬
Na2CO3+2HClØT2NaCl+H2O+CO2”ü£»
106 73 117 44
x y z 4.4g
$\frac{106}{44}$=$\frac{x}{4.4g}$ x=10.6g
$\frac{73}{y}$=$\frac{117}{z}$=$\frac{44}{4.4g}$
y=7.3g
z=11.7g
»ģŗĻĪļÖŠĀČ»ÆÄĘŗĶĢ¼ĖįÄĘÖŹĮæµÄ×ī¼ņÕūŹż±Č£ŗ£Ø18.9g-10.6g£©£ŗ10.6g=83£ŗ106£»
Õō·¢ŗóĖłµĆČÜŅŗµÄČÜÖŹÖŹĮæ·ÖŹżĪŖ$\frac{18.9g-10.6g+11.7g}{18.9g+185.5g+100g-4.4g-100g}$”Į100%=10%
ČōÓĆ36.5%µÄÅØŃĪĖįÅäÖĘŹµŃéĖłÓƵÄĻ”ŃĪĖįÓƵ½µÄČōÓĆ36.5%µÄÅØŃĪĖįµÄÖŹĮæĪŖ7.3g”Ā36.5%=20g
Ōņ¼ÓČėµÄĖ®µÄÖŹĮæĪŖ100g-20g=80g
¹Ź“š°øĪŖ£ŗ
£Ø1£©Na2CO3+2HClØT2NaCl+H2O+CO2”ü
£Ø2£©$\frac{106}{44}$=$\frac{x}{4.4g}$
£Ø3£©83£ŗ106
£Ø4£©10%£®
£Ø5£©80g£®
µćĘĄ øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖ揱£¬µŚŅ»ŅŖÕżČ·ŹéŠ“»Æѧ·½³ĢŹ½£¬µŚ¶žŅŖŹ¹ÓĆÕżČ·µÄŹż¾Ż£¬µŚČż¼ĘĖć¹ż³ĢŅŖĶźÕū£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪŖĮĖ·ĄÖ¹“óĘųĪŪČ¾Ó¦¼õÉŁŹ¹ÓĆ»ÆŹÆČ¼ĮĻ | |
| B£® | ĪŖĮĖ·ĄÖ¹¹āĪŪČ¾Ó¦¾”æÉÄܲ»Ź¹ÓĆ²£Į§Ä»Ē½ | |
| C£® | ĪŖĮĖ·ĄÖ¹Ė®ĢåĪŪČ¾Ó¦½ūÖ¹Ź¹ÓĆÅ©Ņ©»Æ·Ź | |
| D£® | ĪŖĮĖ·ĄÖ¹“óĘųĪŪČ¾Ó¦½ūÖ¹·ŁÉÕ½ÕøŃ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | æÕĘųŹĒŅ»ÖÖ±¦¹óµÄ׏Ō“£¬ĘäÖŠµŖĘųŌ¼Õ¼æÕĘų×ÜĢå»żµÄ78% | |
| B£® | ÄæĒ°ČĖĆĒŅŃÖĘµĆ“æ½šŹō90ÓąÖÖ£¬ĘäÖŠĢśŌŚ“ŗĒļÕ½¹śŹ±ĘŚ¾ĶŌŚĪŅ¹śæŖŹ¼Éś²śŗĶŹ¹ÓĆ | |
| C£® | ŹÆÓĶ·ÖĮóÄܵƵ½ĘūÓĶ”¢ĆŗÓĶ”¢²ńÓĶŗĶĆŗ½¹ÓĶµČČ¼ĮĻ | |
| D£® | µŲĒņÉĻµÄĖ®×ŹŌ“ŹĒ·įø»µÄ£¬µĖ®×ŹŌ“Č“ŗÜÉŁÖ»Ō¼Õ¼Č«ĒņĖ®“¢ĮæµÄ2.53% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ³ĒŹŠÖŠøß“ó½ØÖžĪļµÄ²£Į§Ä»Ē½»įŌģ³É”°¹āĪŪČ¾”° | |
| B£® | ”±ĪĀŹŅŠ§Ó¦”°¼Ó¾ēÖ÷ŅŖŌŅņŹĒ“óĘųÖŠ¶žŃõ»ÆĢ¼µČĘųĢåŗ¬ĮæÉżøßŅżĘšµÄ | |
| C£® | ¼ÓĖŁĮĖŹÆÓĶµÄæŖ²É£¬æÉŅŌæģĖŁĢįøßČĖĄąµÄÉś»īÖŹĮæ | |
| D£® | ĪŖĮĖ·ĄÖ¹Ė®ĪŪČ¾£¬Å©ŅµÉĻŅŖŗĻĄķŹ¹ÓĆ»Æ·Ź”¢Å©Ņ© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
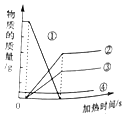
| A£® | øĆ·“Ó¦ÖŠ¢ŁČ«²æ²Ī¼ÓĮĖ·“Ó¦ | |
| B£® | ¢ŚŗĶ¢ŪŅ»¶ØŹĒÉś³ÉĪļ | |
| C£® | ¢ÜæÉÄÜŹĒ“߻ƼĮ | |
| D£® | ·“Ó¦ŗ󣬲Ī¼Ó·“Ó¦µÄ¢ŁµÄÖŹĮæµČÓŚÉś³ÉµÄ¢Ś¢Ū¢ÜµÄÖŹĮæÖ®ŗĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
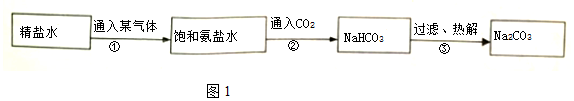
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com