
| ʵ�鲽�� | ʵ������ |
| �ټ��� | Ũ��������Ϊ ˮ�����Ϊ ��д��������̣� |
| ����ȡ | ��Ͳ���� |
| ���ܽ� | һ��Ҫ�Ȱ� |
| 80 |
| x |
| 98 |
| 6g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CuO��Cu��CuO |
| B��O2��Fe3O4��O2 |
| C��NaOH��H2O��NaOH |
| D��Fe��FeCl2��Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������Һ�������¶�ʱ��һ���ܼ����ܽ����� |
| B���ı�������ʹ������Һ�Ͳ�������Һ֮���ת�� |
| C���κ����ʵı�����Һ�����¶Ƚ���ʱ��һ������������ |
| D��һ�����ʵı�����Һ�У��������ܽ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
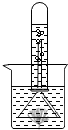 ��ͼ��ʾ����֤��ֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ�װ���뱭ˮ���ձ�����һЩ�����壬��ͨ��һ����������A��ֹһ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ���һ������Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣺
��ͼ��ʾ����֤��ֲ����й�����õ�ʵ��װ�ã�ȡһ���ձ�װ���뱭ˮ���ձ�����һЩ�����壬��ͨ��һ����������A��ֹһ��ʱ�����©����ס�����壬Ȼ��ʢ��ˮ���Թܵ�����©���ϣ���һ������Թ������������ݲ���������Һ���½���������һʵ�飬�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
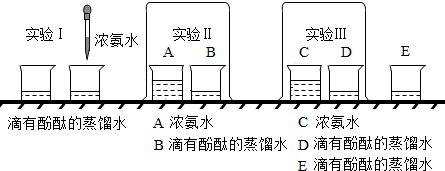
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
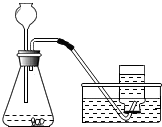 ������̼��ʵ�����Ʒ�
������̼��ʵ�����Ʒ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
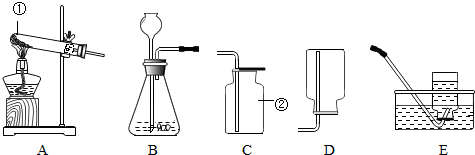
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com