
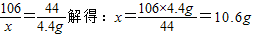
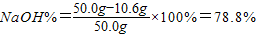


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�챱���ж������п�һģ��ѧ�Ծ��������棩 ���ͣ�������
(4��)Ϊ�ⶨһƿ�����Ѿõ��ռ���NaOH������ijͬѧȡ�������ռ���Ʒ������һ������ˮ���õ�200g��Һ���ټ���200gϡ����(����)��ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g��������ش�
(1)��Ӧ������CO2������Ϊ g��
(2)����ȡ��ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣�
(3)����������ȵ��ռ����ݣ�����һ��ʱ�����һ��δ���ʡ�һ�ݲ��ֱ��ʡ�һ��ȫ������(ע�����ʺ�����ʶ�ΪNa2CO3)�����ֱ�����ͬ���������������ַ�Ӧʱ����Ҫ�����������ȡ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ���� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com